FDA: Page 13
-
FDA finalizes guidance on studying opioid use disorder treatments
The Center for Devices and Radiological Health is aiming to help companies manage challenges such as inaccurate participant reports of drug use.
By Nick Paul Taylor • July 12, 2024 -
Deep Dive
FDA’s lab developed test rule could be first check on agency’s power post-Chevron
The Supreme Court’s decision to overturn the Chevron doctrine would make it easier to challenge agency regulations, such as the LDT final rule.
By Susan Kelly , Elise Reuter • July 11, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
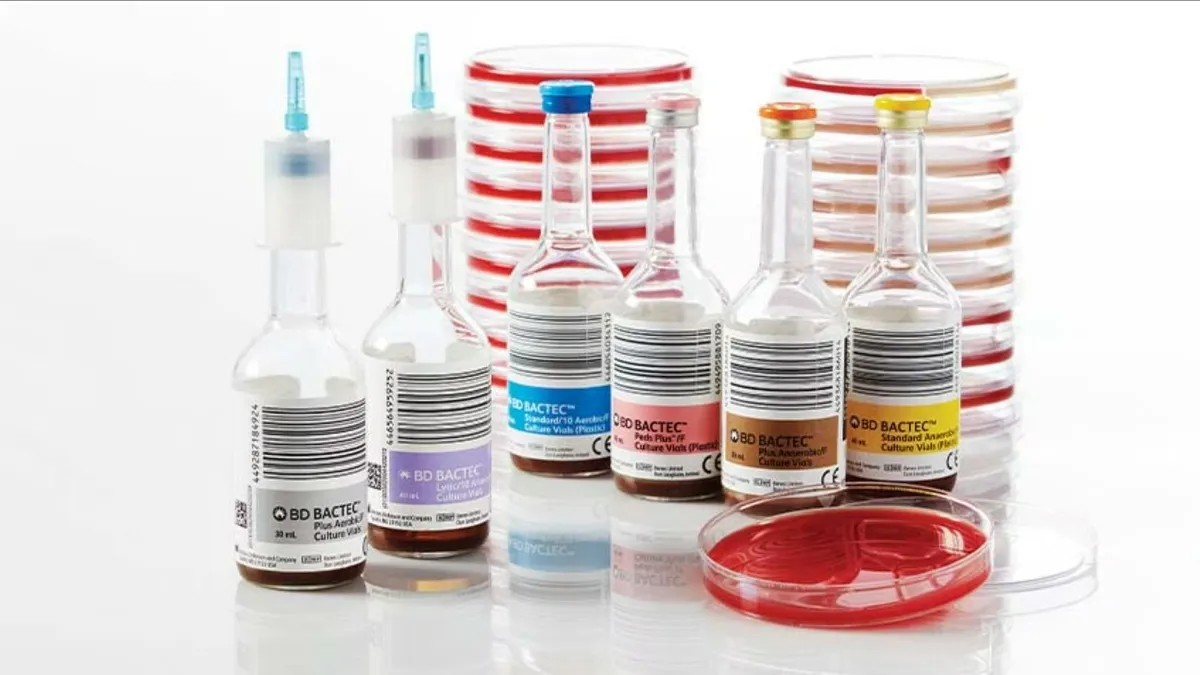
FDA warns BD blood culture bottle shortage could impact diagnosis
BD said reduced availability of blood culture media bottles due to supplier issues has prevented it from meeting global demand.
By Nick Paul Taylor • July 11, 2024 -
Medtronic recalls endotracheal tubes over blockage risk
The Food and Drug Administration has told healthcare providers to stop using the two affected types of endotracheal tubes.
By Nick Paul Taylor • July 11, 2024 -
Federal officials step down from health AI group’s board
HHS officials Micky Tripathi and Troy Tazbaz resigned from their roles as non-voting members of the Coalition for Health AI, an industry group working to create standards for artificial intelligence in healthcare.
By Emily Olsen • July 10, 2024 -
Roche wins CE mark for its first CGM
The clearance positions Roche to challenge Abbott and Dexcom for the European continuous glucose monitor market.
By Nick Paul Taylor • Updated July 10, 2024 -
Pulse drops 510(k) plan for PFA device after FDA requests clinical data
Analysts said the AFib device is now unlikely to come to market before late 2026.
By Nick Paul Taylor • July 9, 2024 -

 Retrieved from Cardinal Health on July 02, 2024
Retrieved from Cardinal Health on July 02, 2024
FDA continues crackdown on plastic syringes made in China
The agency has issued import bans for four manufacturers, and multiple companies have recalled affected syringes. Check out MedTech Dive’s roundup of the news.
By Elise Reuter • July 2, 2024 -
FDA to expand total product life cycle program
Officials plan to start accepting radiological and ophthalmic medical devices into the program, designed to accelerate medtech innovation, in October.
By Nick Paul Taylor • July 2, 2024 -
FDA shares draft guidance on clinical trial diversity action plans
The agency said which trials need diversity action plans and presented the timeline for implementing the requirement.
By Nick Paul Taylor • June 28, 2024 -
Cepheid wins FDA nod for fingerstick hepatitis C test
Patients could be diagnosed and potentially receive treatment at the same healthcare visit.
By Nick Paul Taylor • June 28, 2024 -
FDA partners with Gates Foundation to support breath-based diagnostic development
Through a $1.9 million Gates Foundation grant, the collaborators will create methods to help developers make tests that can easily be deployed to rural and remote areas.
By Nick Paul Taylor • June 27, 2024 -
Cardinal Health recalls procedure kits over plastic syringes made in China
Cardinal listed more than 500 catalog numbers in its recall notice and told customers to remove and discard the syringes.
By Nick Paul Taylor • June 24, 2024 -
Q&A
ONC’s Micky Tripathi on laying the digital floor for healthcare AI
The agency head discussed ONC’s accomplishments over the past two decades, artificial intelligence opportunities and improving the documentation burden among clinicians.
By Emily Olsen • June 20, 2024 -
FDA official sets out approach to AI in medical devices
Developing quality assurance practices for AI models should be a priority, said Troy Tazbaz, director of the CDRH’s Digital Health Center of Excellence.
By Elise Reuter • June 18, 2024 -
Senators urge CMS to create AI payment pathway
Uniform reimbursement standards are needed to protect patient access to algorithm-based devices and ensure future innovation, the lawmakers said.
By Nick Paul Taylor • June 18, 2024 -
FDA creates transparency principles for AI in medical devices
The agency worked with regulators in the U.K. and Canada to determine how key information about machine learning-enabled devices should be communicated.
By Nick Paul Taylor • June 17, 2024 -
Medtech group updates EtO standard for first time in 25 years
The Association for the Advancement of Medical Instrumentation said the changes incorporate new technologies and address industry challenges.
By Elise Reuter • June 14, 2024 -
Teleflex catheter kit recall linked to 31 injuries, 3 deaths
Teleflex reported 322 complaints about a safety problem that can prevent the balloon from fully inflating.
By Nick Paul Taylor • June 14, 2024 -
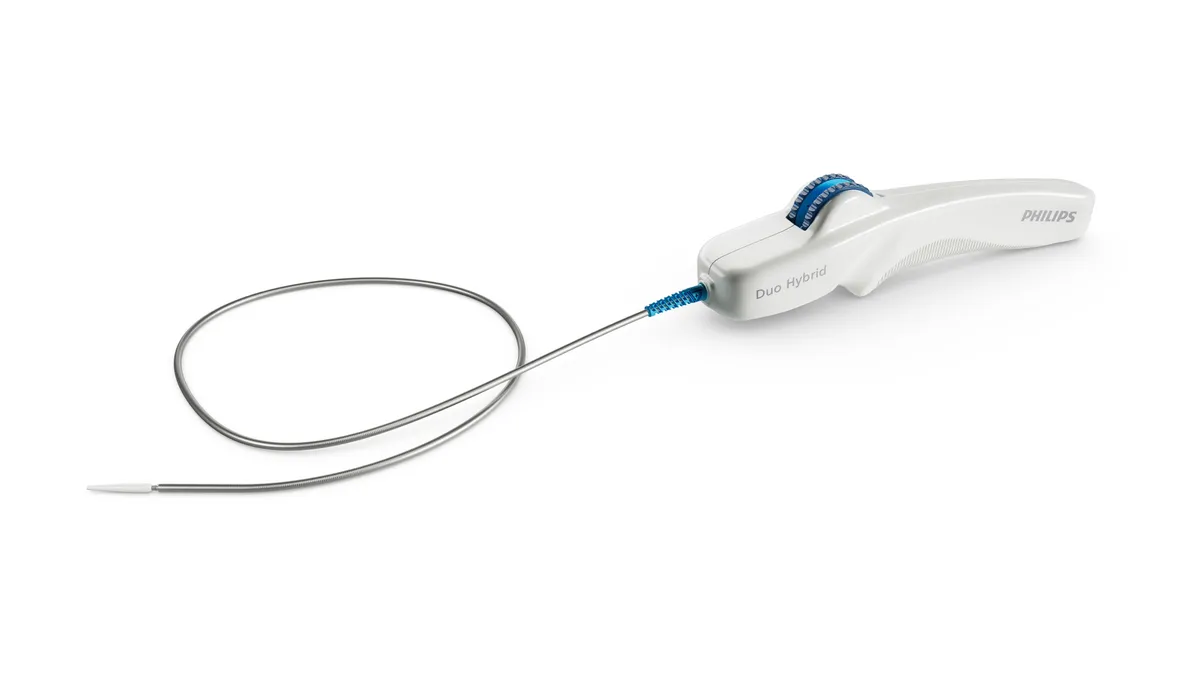
Philips launches stent system to restore blood flow in blocked veins
Philips acquired the device as part of its 227 million euro purchase of Vesper Medical in 2022.
By Nick Paul Taylor • June 13, 2024 -
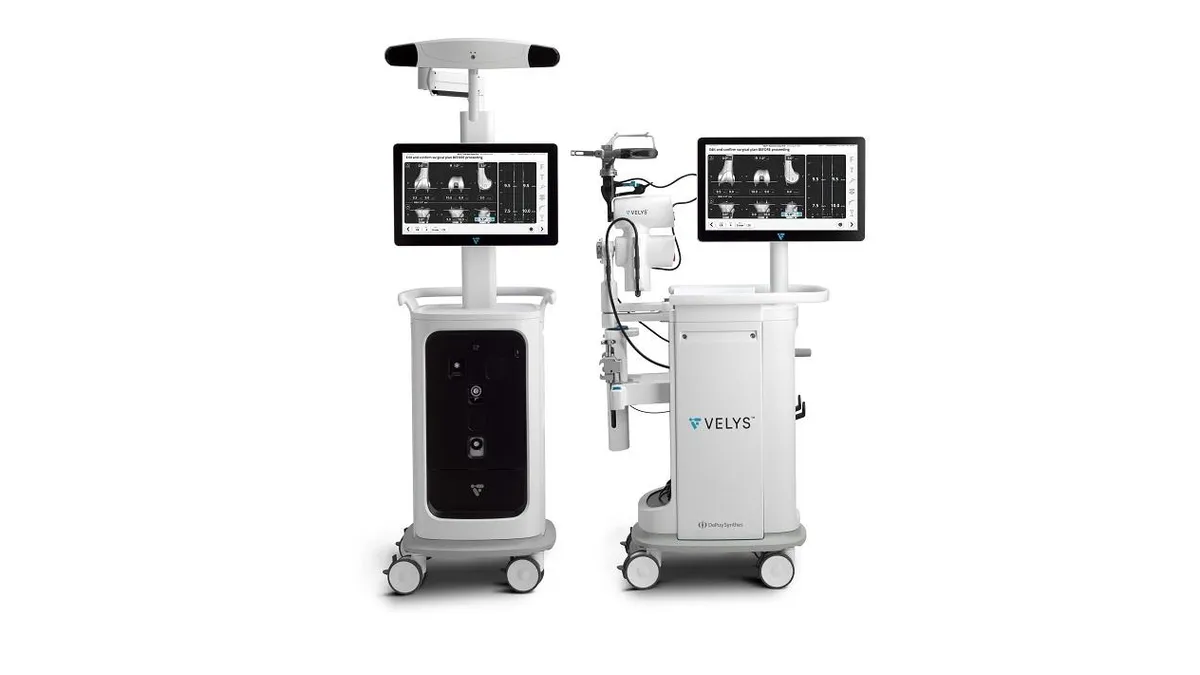
J&J wins expanded FDA clearance for knee surgery robot
Johnson & Johnson has identified Velys as a product that can help it regain market share from rivals such as Stryker.
By Nick Paul Taylor • June 10, 2024 -
Abbott wins CE mark for dual-chamber leadless pacemaker
Abbott sees the device as a product that can help its rhythm management business grow 6% to 7%.
By Nick Paul Taylor • June 7, 2024 -
Q&A
Stronger oversight of AI needed in medical devices, ECRI CEO says
Marcus Schabacker called for more upfront regulations and postmarket monitoring to better understand how AI features affect patient care.
By Elise Reuter • June 5, 2024 -
Lab industry sues to stop FDA’s new LDT rule
The American Clinical Laboratory Association argues the agency does not have the authority to enforce stricter standards on lab developed tests.
By Susan Kelly • May 30, 2024 -
New products take center stage in medtech’s latest earnings season
From pulsed field ablation devices to Intuitive's latest da Vinci robot, product launches dominated recent earnings calls.
By Ricky Zipp • May 28, 2024