FDA: Page 21
-
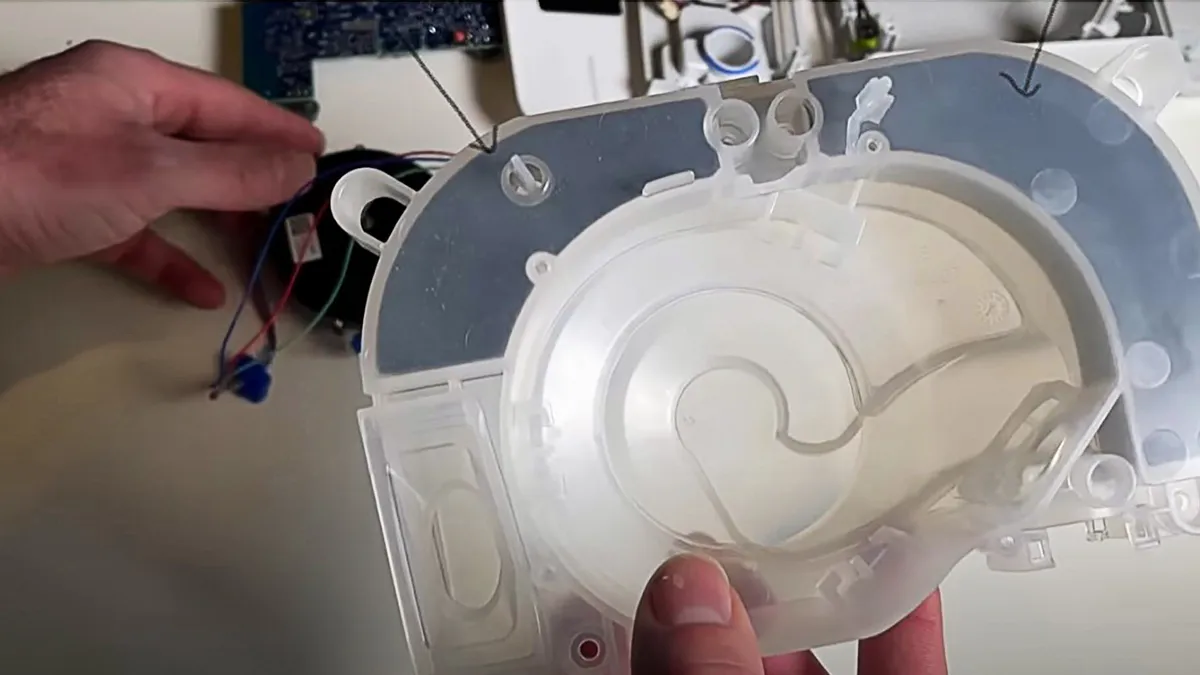
FDA warning letter accuses Wavi of selling unapproved neurological device
Wavi markets its headset as providing a baseline scan for patients who have a traumatic event, an injury or are worried about their brain as they age.
By Nick Paul Taylor • Nov. 1, 2023 -
Tracker
Tracking Philips Respironics recalls
Philips recalled its Trilogy ventilators to fix new and previously reported problems with the machines. The company issued a mandatory software update and changed the devices’ instructions.
By Elise Reuter • Updated Oct. 3, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA posts updated safety data on Bayer’s Essure, notes progress on improving study
The agency removed the “inadequate” progress tag it applied last year after Bayer acted to get people back in the study.
By Nick Paul Taylor • Oct. 31, 2023 -
Philips leader signed off on sale of respiratory devices despite evidence of health risks: report
Roy Jakobs approved the sale of respiratory devices held by manufacturers after the company stopped shipping new machines, according to an article published last week by Pittsburgh Post-Gazette and ProPublica.
By Nick Paul Taylor • Oct. 30, 2023 -
Fresenius’ work to replace hemodialysis tubing flagged as Class I recall by FDA
Agency officials warned healthcare professionals last year about the potential for silicone tubing to expose patients to toxic compounds.
By Nick Paul Taylor • Oct. 25, 2023 -
5 takeaways from the FDA’s updated list of cleared AI/ML medical devices
The agency recently added 171 new devices to its list, with radiology once again accounting for the most authorizations.
By Elise Reuter • Oct. 24, 2023 -
US trade commission explores impact of patent rules on COVID diagnostics
The USITC report presents conflicting testimony from the medtech industry and advocacy groups about the impact of compulsory licensing.
By Nick Paul Taylor • Oct. 23, 2023 -
AdvaMed CEO warns Congress of supply shortages from EtO, PFAS regulations
The trade group leader called for changes to the EPA’s proposals, while a scientist with UCSF said lawmakers should turn to science free of vested financial interests.
By Elise Reuter • Oct. 20, 2023 -
Supply disruptions are delaying surgeries and compromising patient care, survey finds
A patient safety nonprofit used the findings to call for “long-term, nationally coordinated solutions” to stop persistent shortages.
By Nick Paul Taylor • Oct. 19, 2023 -
FDA finalizes COVID-era guidance, extending flexibility for some remote monitoring devices
The agency shortened its list of devices that can continue to undergo limited modifications without 510(k) filings.
By Nick Paul Taylor • Oct. 19, 2023 -
GE HealthCare cuts formaldehyde levels in neonatal incubators with revised process
The FDA previously alerted healthcare providers to the potential for neonatal incubators to emit airborne chemicals.
By Nick Paul Taylor • Oct. 17, 2023 -
FDA: ‘We have had a very loud voice’ on EtO
The agency said it is working closely with the Environmental Protection Agency, which is slated to implement a final rule in March limiting ethylene oxide emissions for medical device sterilizers.
By Elise Reuter • Oct. 16, 2023 -
Masimo wins de novo authorization for measure of high blood oxygen levels
The ORi device is intended to support standard pulse oximetry by providing additional insights into patients with higher than normal oxygenation.
By Nick Paul Taylor • Oct. 16, 2023 -
J&J receives 510(k) clearance for foot fixation device, targets growing bunion market
The clearance comes shortly after Stryker introduced a minimally invasive system for treating bunions.
By Nick Paul Taylor • Oct. 16, 2023 -
Illumina details EC timeline, funding requirements for Grail divestment
The European Commission ordered the gene sequencing company to support Grail with two-and-a-half years of funding if it pursues a spinoff.
By Susan Kelly • Oct. 13, 2023 -
FDA prioritizes guidance on AI, cybersecurity, pulse oximeters in stacked schedule for 2024
The administration is ramping up production of medtech guidance, adding 18 draft documents to the list of priorities for the upcoming financial year.
By Nick Paul Taylor • Oct. 13, 2023 -
European Commission orders Illumina to divest Grail
The order includes an undisclosed timeline and potential additional fines. Illumina, which has been anticipating the decision, maintains the commission does not have jurisdiction over the completed merger.
By Susan Kelly • Oct. 12, 2023 -
Boston Scientific wins diabetic neuropathy approval, joining rivals in growing market
The expanded indication positions Boston Scientific to challenge Abbott, Medtronic and Nevro for the emerging opportunity in spinal cord stimulation to treat the condition.
By Nick Paul Taylor • Oct. 12, 2023 -
FDA to establish digital health advisory committee
The agency is seeking advisers to help it understand the benefits, risks and outcomes associated with digital technologies.
By Elise Reuter • Oct. 12, 2023 -
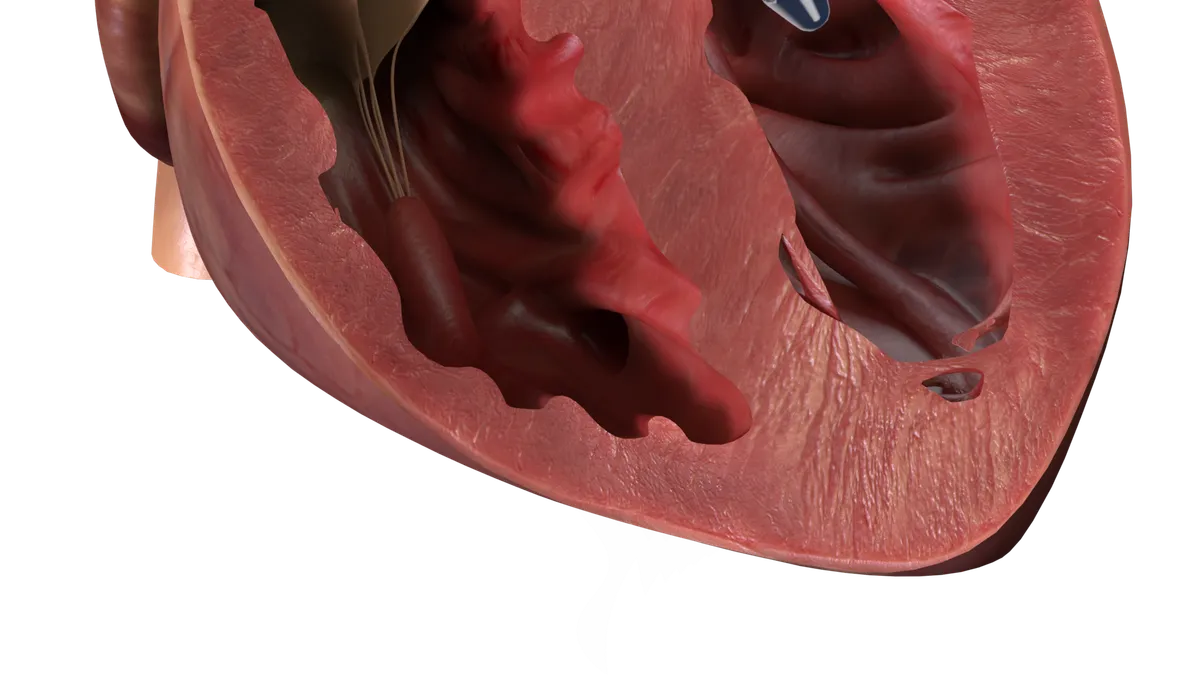
J&J’s Abiomed hit with FDA warning letter over Impella heart pump
The letter identifies quality system problems in a group of recalled devices and says monitoring software used with the pump requires premarket authorization.
By Susan Kelly • Oct. 12, 2023 -
AdvaMed elevates digital health center to division, names board
The new division will focus on four core policy priorities under Chair Taha Kass-Hout, chief technology officer at GE HealthCare.
By Susan Kelly • Oct. 10, 2023 -
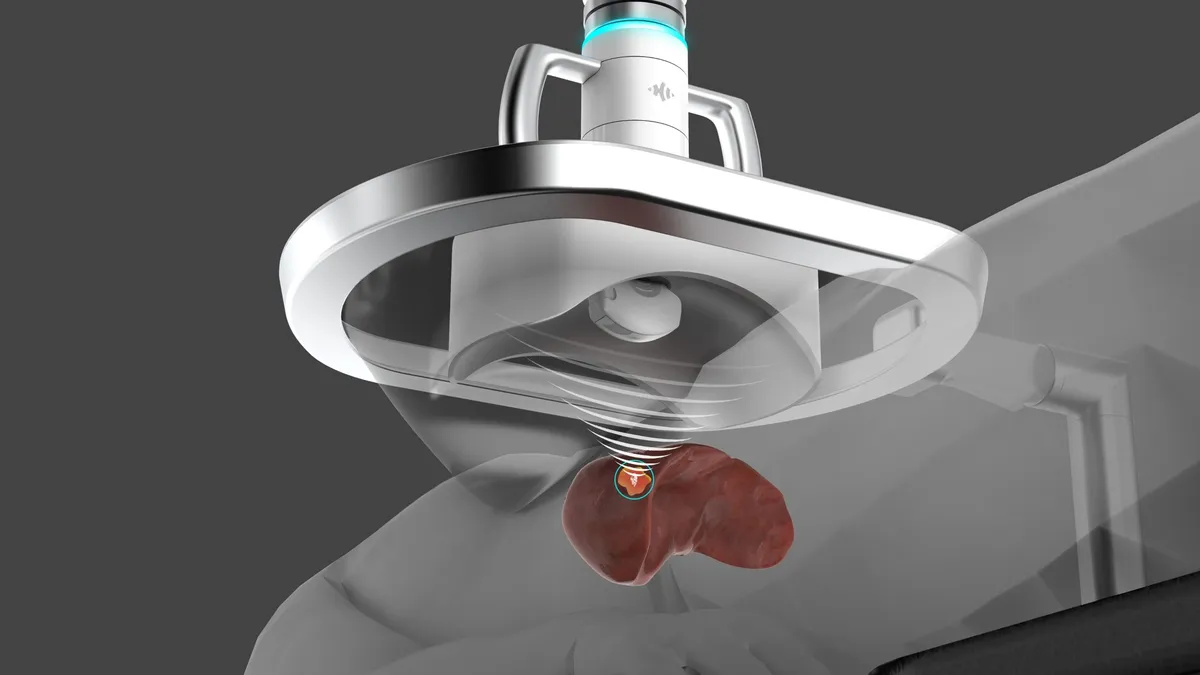
J&J-backed HistoSonics gets de novo clearance for liver cancer treatment
The system offers a minimally invasive alternative to surgical resection and thermal ablation in patients with primary and metastatic liver cancers.
By Nick Paul Taylor • Oct. 10, 2023 -
EU poised to mandate Illumina sell Grail liquid biopsy unit: FT
Regulators could order the divestment of the business as soon as next week to punish Illumina for defying their authority and “illegally” acquiring Grail.
By Nick Paul Taylor • Oct. 10, 2023 -
Invitae test for hereditary cancers gains FDA de novo nod
The test analyzes DNA from a single blood sample to identify hundreds of variants in 47 genes associated with predispositions for a range of cancer types.
By Susan Kelly • Oct. 3, 2023 -
FDA expands total product life cycle program to cover neurological devices
The agency could enroll up to 45 additional devices over the coming year.
By Nick Paul Taylor • Oct. 3, 2023