FDA: Page 26
-
Great Britain proposes allowing CE marks until 2030 to minimize Brexit disruption
The extended timeline to create a new regulatory system in England, Scotland and Wales is intended to prevent medical device shortages.
By Nick Paul Taylor • May 1, 2023 -
Illumina gets cybersecurity warning from FDA over sequencing software
The vulnerability could result in an attacker gaining control of an instrument remotely and altering genomic data results, the agency said.
By Susan Kelly • Updated April 28, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
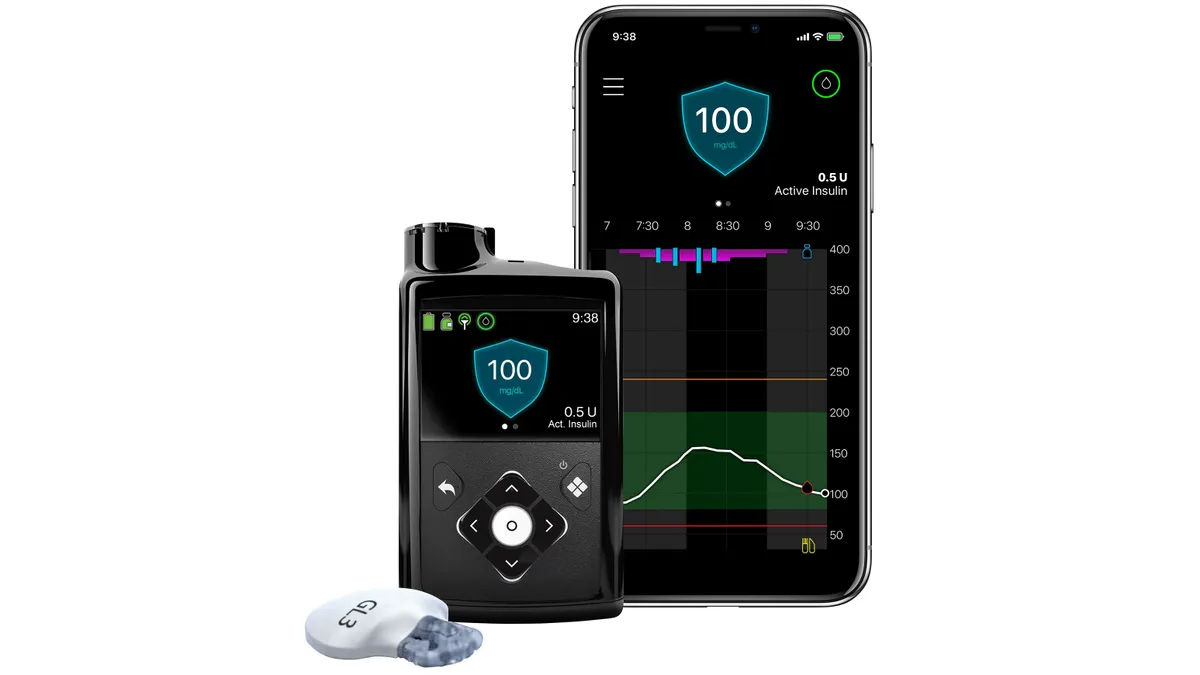
Medtronic diabetes warning letter lifted by FDA
The resolution will allow Medtronic to bring new products to market faster, such as its new continuous glucose monitor, Simplera.
By Elise Reuter • April 25, 2023 -
Medtronic wins FDA approval for long-delayed MiniMed 780G insulin pump
Analysts called the FDA approval a “positive” for Medtronic that “should somewhat aid in a turnaround of its U.S. diabetes franchise.”
By Nick Paul Taylor • April 24, 2023 -
Supreme Court maintains access to abortion pill, blocking restrictions on its use
The decisions stayed by the Supreme Court could affect future challenges to the FDA’s authority to regulate drugs and medical devices.
By Delilah Alvarado • Updated April 22, 2023 -
Report names connected medical devices with the biggest cybersecurity risks
The findings come as medical device makers face upcoming FDA requirements for providing cybersecurity information as part of their pre-market submissions.
By Nick Paul Taylor • April 21, 2023 -
Opinion
Ending free COVID tests risks worsening the pandemic
If Americans start paying out of pocket for COVID tests, they'll test much less — if at all, argue representatives of the Testing at Home Coalition.
By Amy Kelbick and Eric Zimmerman • April 20, 2023 -
Abbott hit with FDA warning letter over unapproved changes to heart disease test
The FDA said that four changes made to the tests “could significantly affect the safety or effectiveness of the devices.”
By Nick Paul Taylor • Updated April 20, 2023 -
Fresenius flags fault with infusion system acquired last year in $240M deal
Fresenius noted a problem with the devices that can cause loss of power, potentially delaying or interrupting the supply of critical fluids and medications.
By Nick Paul Taylor • April 20, 2023 -
Post-approval modification of high-risk devices linked to 30% jump in recall risk: study
The authors suggest improved post-marketing surveillance systems may be needed to mitigate risks to patient safety.
By Nick Paul Taylor • April 18, 2023 -
Zimmer’s ‘smart knee’ implant may qualify for new technology payments from CMS
If finalized, the add-on payments would help cover the cost of the sensor technology included in the company’s Persona IQ knee implants.
By Elise Reuter • April 12, 2023 -
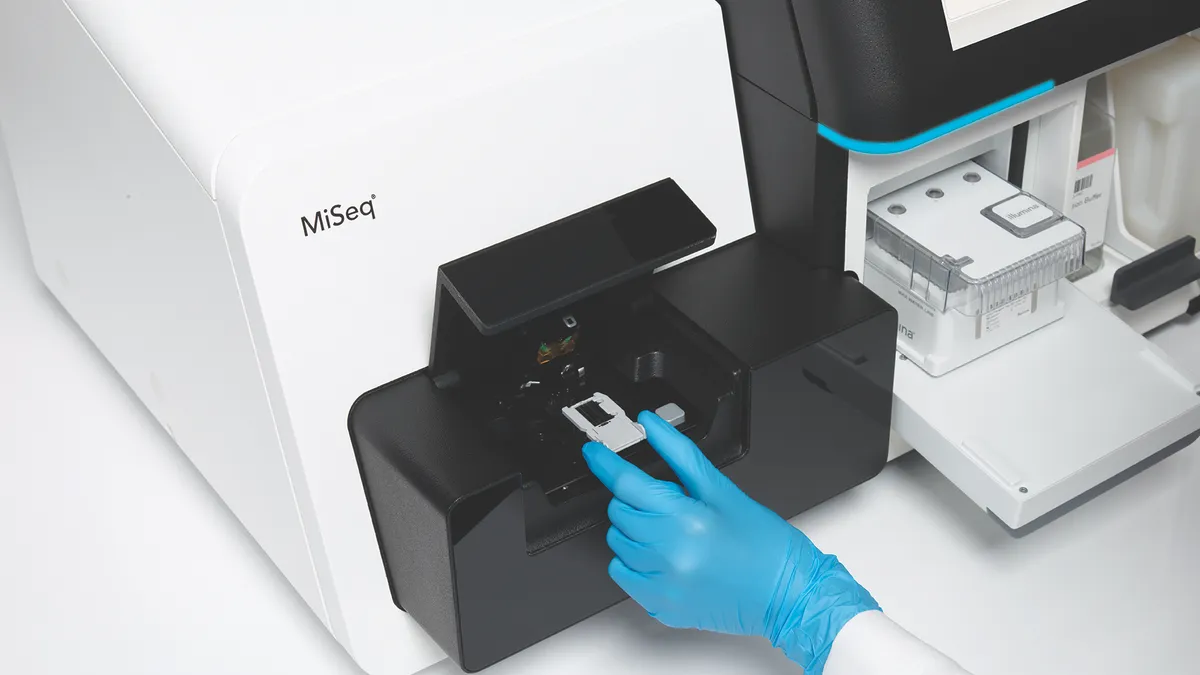
Illumina-Icahn proxy battle heats up as Grail divestment orders loom
DNA-sequencing provider Illumina is headed for a showdown with billionaire financier Carl Icahn over three of nine seats on its board of directors as it continues to defy regulators’ orders to divest cancer test maker Grail.
By Susan Kelly • April 12, 2023 -
FDA starts device sterilization pilot program to help industry adapt to EtO emission clampdown
The FDA “recognizes the need to facilitate more timely changes” amid a push to cut EtO emissions and constraints on the supply of radioactive cobalt.
By Nick Paul Taylor • April 12, 2023 -
Icahn pushes for spinoff of Illumina’s Grail liquid biopsy unit
The billionaire financier, who is waging a proxy fight for seats on Illumina’s board, is arguing that Grail should become a standalone publicly traded company.
By Susan Kelly • April 11, 2023 -
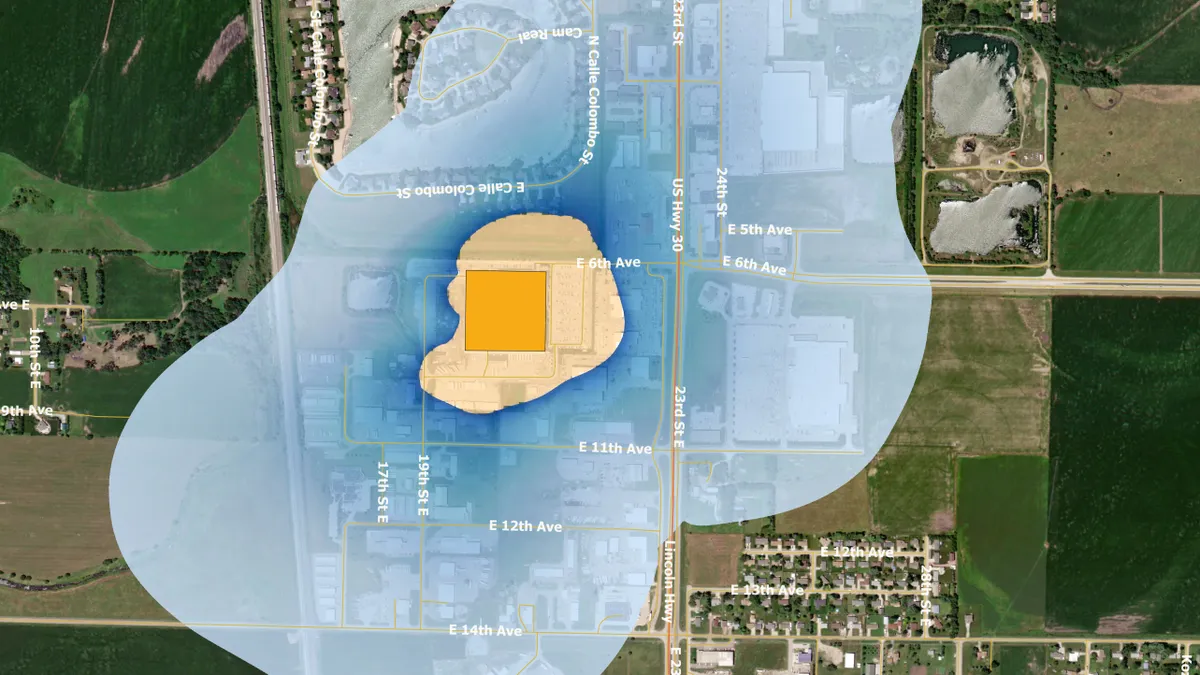
 EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
Medical device groups say ‘the stakes are high’ with new EtO regulations
AdvaMed, which represents medical device companies, warned that patients could face treatment delays if sterilization facilities close.
By Elise Reuter • April 11, 2023 -
EPA to limit ethylene oxide emissions from medical device sterilizers
The agency proposed new emissions limits that device sterilization companies would have 18 months to meet.
By Elise Reuter • April 11, 2023 -
Illumina backs its board in proxy fight with Icahn
Illumina directors up for re-election to one-year terms include prominent leaders in healthcare such as former FDA Commissioner Scott Gottlieb and longtime Intuitive Surgical CEO Gary Guthart.
By Susan Kelly • April 10, 2023 -
Philips recalls 1,200 reworked sleep apnea devices over fault that can cause therapy failure
The problems keep mounting for Philips, as recently repaired respirators now need to be fixed in a recall the FDA has labeled a Class I event.
By Nick Paul Taylor • April 10, 2023 -
EPA proposes stricter ethylene oxide pollutant rules for chemical makers
Further EPA proposals involving medical device sterilization plants are expected to be forthcoming.
By Susan Kelly • April 7, 2023 -
Diagnostic testing reform bill has a path to the finish line this year: AdvaMed
Legislators recently reintroduced the VALID Act, which would bring lab-developed tests and in-vitro diagnostics under one framework, after failing to pass the bill twice last year.
By Elise Reuter • Updated April 5, 2023 -
Why won’t Illumina sell Grail?
While financier Carl Icahn and regulators in the U.S. and Europe want DNA-sequencing company Illumina to divest liquid biopsy company Grail, Illumina’s board won’t budge. Analysts say they may be waiting for the best moment.
By Susan Kelly • April 4, 2023 -
Masimo receives De Novo nod from FDA for device designed to detect signs of opioid overdose
The system detects patterns associated with opioid-induced respiratory depression and sends alerts that escalate in line with the risk level.
By Nick Paul Taylor • April 4, 2023 -
Illumina ordered to divest Grail by FTC on anticompetition concerns
The San Diego-based DNA-sequencing company, which also faces a challenge from European antitrust regulators, said it will appeal the FTC’s order.
By Susan Kelly • April 3, 2023 -
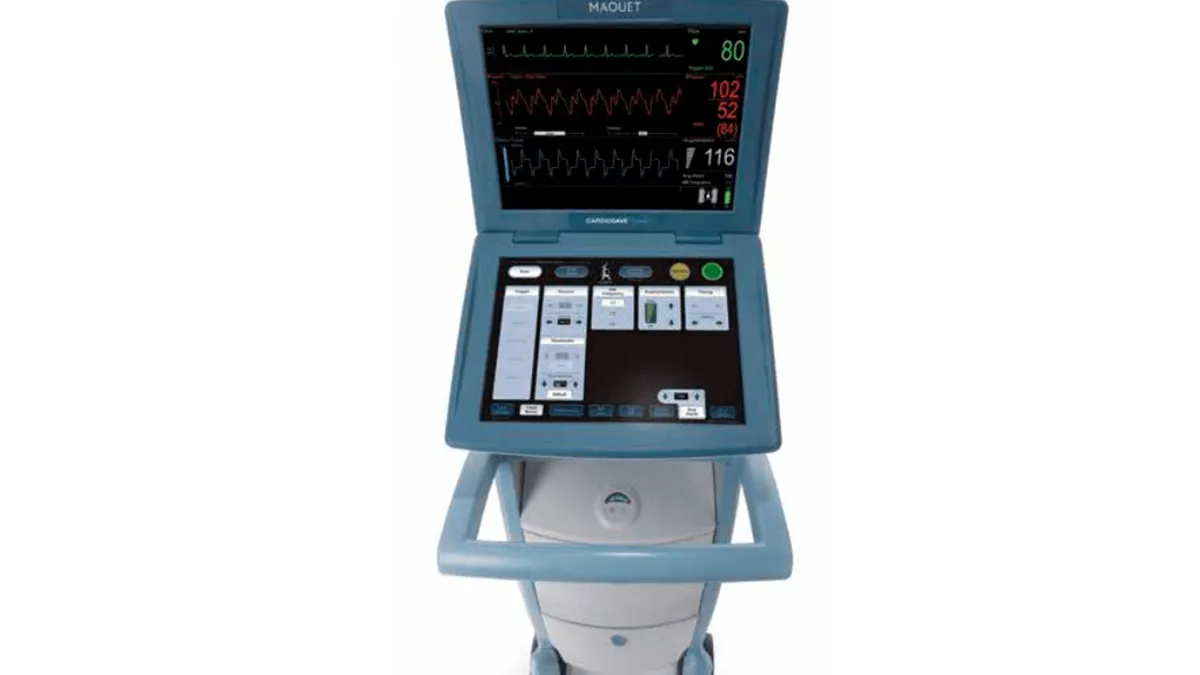
Getinge’s quality woes continue: FDA categorizes another heart pump recall as Class I event
The recall is the latest in a series of compliance problems for Getinge, which has had CE marks for two products suspended.
By Nick Paul Taylor • April 3, 2023 -
Illumina urges shareholders to reject Icahn board nominees
The DNA-sequencing company says Icahn’s board nominees don’t understand its business or the regulatory process.
By Susan Kelly • March 31, 2023