FDA: Page 31
-
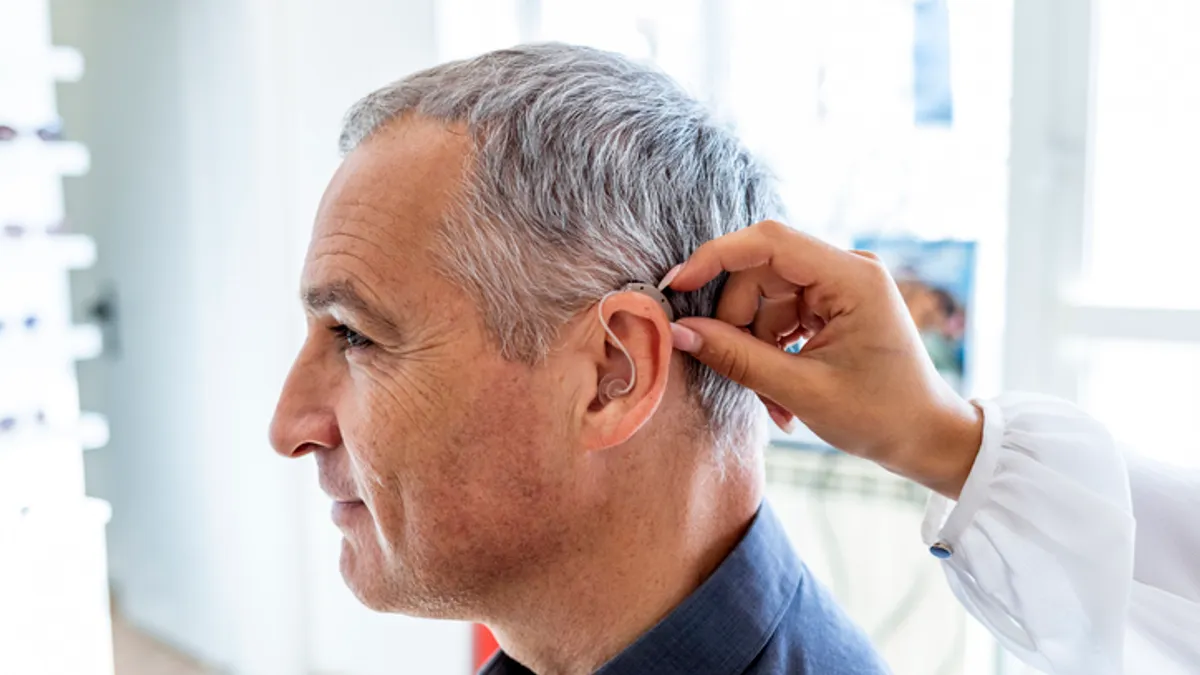
Bose-partnered Lexie to launch $999 OTC hearing aid, challenging Sony, Eargo in nascent market
Lexie has matched the price of the first Sony device and is seeking to differentiate itself through the use of a rechargeable battery.
By Nick Paul Taylor • Oct. 14, 2022 -
Sony begins sale of OTC hearing aids with entry-level price of $999
Lower-cost, over-the-counter hearing aids are becoming available to the 70% of older Americans who need the devices but don’t have them.
By Peter Green • Oct. 13, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
 Retrieved from Jiangsu Well Biotech Co. on October 12, 2022
Retrieved from Jiangsu Well Biotech Co. on October 12, 2022
Jiangsu Well Biotech distributed unapproved COVID-19 tests, FDA says
The company is the latest firm to recall rapid antigen tests that had never received an emergency use authorization or clearance from the regulator.
By Elise Reuter • Oct. 12, 2022 -
FDA starts advisory program pilot to reduce ‘valley of death’ risk for medical devices
A plan to ensure more medical devices pass from testing to clinical application aims to help dozens of devices through the approval process each year.
By Nick Paul Taylor • Oct. 12, 2022 -
Software to predict risk of sepsis, stroke should be regulated as a medical device, says FDA
Clarity on rules welcomed by some device makers, who also cautioned that products may take longer to come to market.
By Elise Reuter • Oct. 11, 2022 -
FDA finalizes postapproval study guidance in light of AdvaMed, Foundation Medicine feedback
The agency resisted calls to give sponsors more time to prepare protocols for post-approval studies.
By Nick Paul Taylor • Oct. 11, 2022 -
Abbott lands FDA emergency authorization for first commercial monkeypox test
Abbott’s testing process is fully automated to increase throughput to help control the spread of monkeypox.
By Nick Paul Taylor • Oct. 10, 2022 -
NovaSight’s digital treatment for lazy eye gets FDA nod, providing alternative to patching
A randomized controlled trial found the digital device to be as effective as wearing an eye patch, the current gold standard for treatment, opening the way for more children to complete necessary therapy for amblyopia.
By Nick Paul Taylor • Oct. 10, 2022 -
Owlet seeks clearance of blood-oxygen-measuring baby sock after FDA warning letter
Owlet now is a step closer to selling a prescription medical device designed to alert parents when their baby’s heart rate or oxygen saturation levels move outside of prescribed ranges.
By Nick Paul Taylor • Oct. 10, 2022 -
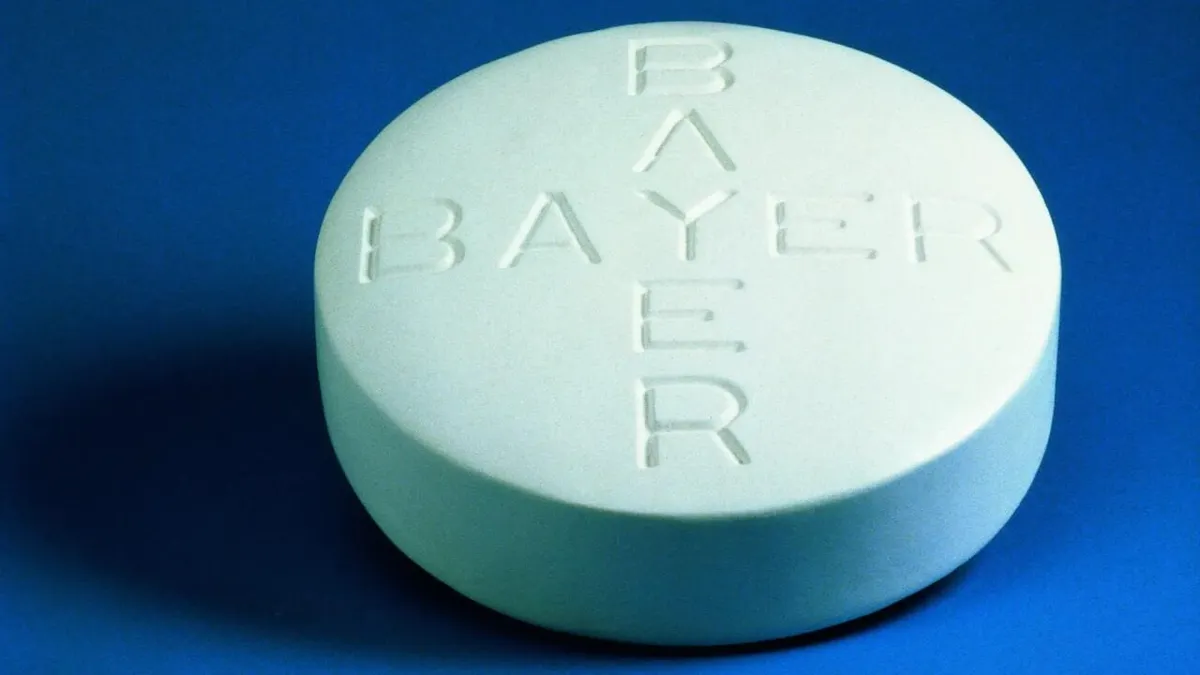
‘Inadequate’ progress of Bayer’s study on birth-control implant called out by FDA as patients drop out
The FDA saw a rise in patients dropping out of a study on the Essure birth-control implant, prompting it to tell Bayer to develop and implement strategies to ensure the work continues.
By Nick Paul Taylor • Oct. 7, 2022 -
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
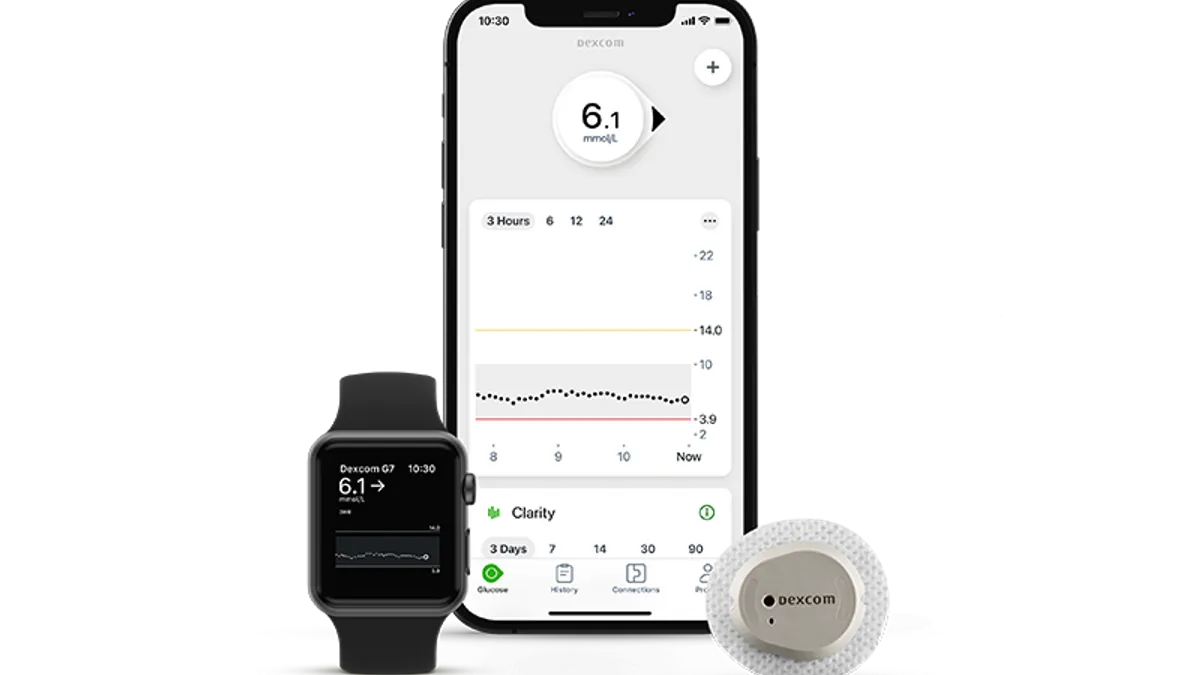
Dexcom, Abbott have ‘massive opportunity’ with new CGM coverage proposal: analysts
Analysts at J.P. Morgan said the proposal reads “very favorably” for the two leading makers of continuous glucose monitors and unlocks “a major near-term driver for growth.”
By Nick Paul Taylor • Oct. 7, 2022 -
BD discloses cybersecurity vulnerability in cervical cytology processing machine
The company is advising users to address the threat by restricting access to the instrument while it works on a software patch.
By Nick Paul Taylor • Oct. 6, 2022 -
Dexcom starts global rollout of G7 CGM system, launching device in U.K. and Germany
Software updates have delayed the introduction of the product in the U.S. at a time when the company seeks to take market share from rival Abbott.
By Nick Paul Taylor • Oct. 6, 2022 -
Roche wins FDA approval for companion diagnostic to support breast cancer therapy
The approval positions Roche to support the rollout of AstraZeneca and Daiichi Sankyo’s Enhertu in HER2-low breast cancer.
By Nick Paul Taylor • Oct. 5, 2022 -
Illumina looks to resolve Grail acquisition ‘sooner rather than later’: analysts
With a divestiture order from the European Commission looming, Illumina is looking at strategic options for the maker of blood tests used to detect cancer.
By Elise Reuter • Oct. 4, 2022 -
LivaNova recall of open-heart-surgery blood pump designated as Class I event by FDA
A software malfunction can mistakenly freeze the machine, set off an alarm that can’t be muted and lead to the heart pump stopping during surgery.
By Nick Paul Taylor • Updated Oct. 12, 2022 -
Medtech groups praise MDUFA passage as legislators plan to include reforms in end-of-year bill
The legislation will provide the FDA with as much as $1.9 billion over the next five years, but changes to diagnostics and medical device cybersecurity were slashed from a "clean version" of the bill.
By Elise Reuter • Oct. 3, 2022 -
European AI Act could have ‘significant impact’ on manufacturers, medtech group warns
“Overregulation and misalignment” could create uncertainty and stop products from coming to market, MedTech Europe says.
By Nick Paul Taylor • Oct. 3, 2022 -
Pulse oximeter bias delayed treatment of Black COVID-19 patients by hours: study
An author of the paper called for manufacturers to “go back to the drawing board to provide clinicians with a tool that is free from bias.”
By Nick Paul Taylor • Sept. 30, 2022 -
Veterans Affairs, FDA partner to accelerate medical device development
The goal is to provide standardized, off-the-shelf tests that can streamline the regulatory process and reduce time to market.
By Nick Paul Taylor • Sept. 30, 2022 -
Congress passes continuing resolution reauthorizing FDA user fees; bill heads to Biden for signature
President Biden is expected to sign last-minute deal to fund the government, that includes a “clean” bill without amendments to fund FDA review activities for five years.
By Elise Reuter • Updated Sept. 30, 2022 -
FDA finalizes guidance on how clinical decision support software is regulated
The FDA has overhauled the 2019 draft, deleting some sections and completely rewriting other parts of the document.
By Nick Paul Taylor • Sept. 28, 2022 -
FDA switches COVID-19 tests to De Novo, 510(k) pathways, limiting EUAs
The FDA will still review EUA requests from “experienced developers” of tests that meet certain criteria.
By Nick Paul Taylor • Sept. 28, 2022 -
FDA user fees package won’t include any amendments, House leaders say
Cybersecurity and clinical trial diversity amendments passed by the U.S. House of Representatives won’t be in the final version of the bill.
By Elise Reuter • Sept. 27, 2022 -
FDA makes case for ‘new regulatory paradigm’ amid hurdles in software-oversight program
The agency seeks to tailor requirements based on the latest science, the benefits and risks posed by devices, their real-world performance and their contribution to promoting health equity.
By Nick Paul Taylor • Sept. 27, 2022