FDA: Page 48
-
Zimmer 'smart knee' gets FDA nod as ortho rivalry with Stryker heats up
The orthopaedics device maker, through a collaboration with Canary Medical, becomes the first company to bring an implantable smart device for total knee replacement to the U.S. market.
By Susan Kelly • Aug. 31, 2021 -

 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Micrograph]. Retrieved from Flickr.
BD gets emergency FDA nod for smartphone-enabled COVID-19 test
The medtech is launching the product against a backdrop of rising demand for similar diagnostics, as the delta wave spurs a surge in cases nationwide after a pullback in the first part of the year.
By Nick Paul Taylor • Aug. 27, 2021 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medtechs made it out of Q2 with minimal impact from delta. That could change in Q3.
After procedure-dependent medtechs reported strong elective returns in the first half of 2021, the delta variant is now posing a risk to the industry's recovery as hospitals once again shut down procedures.
By Ricky Zipp • Aug. 26, 2021 -
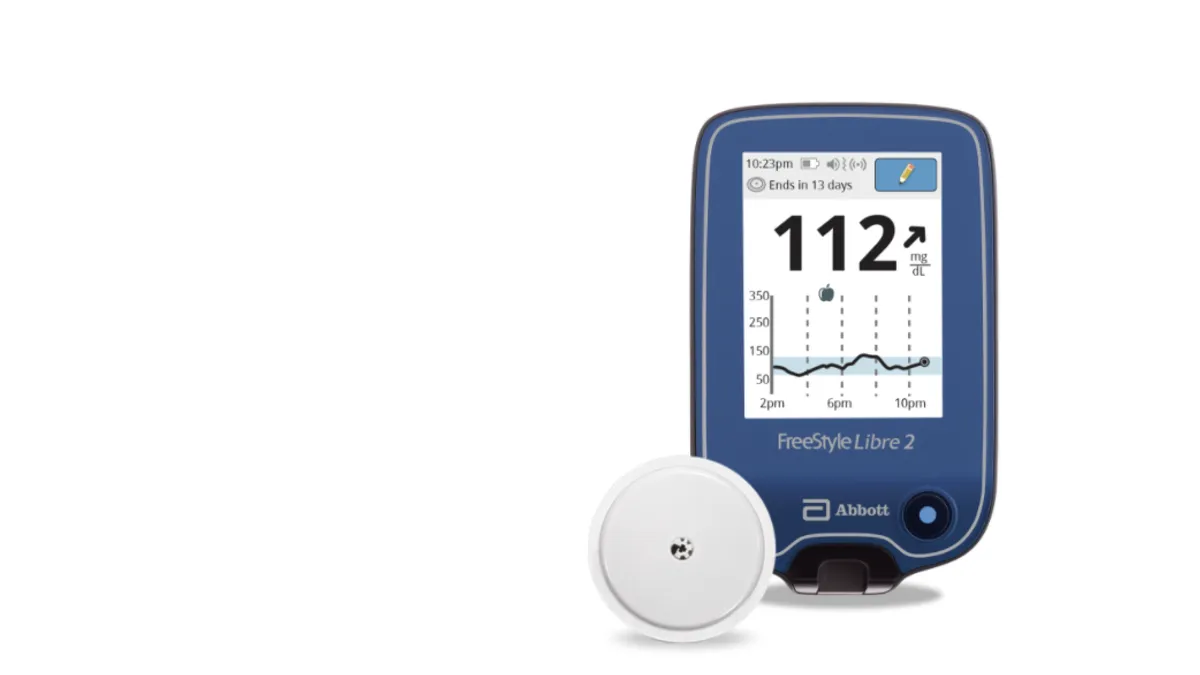
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Diabetes screening advised to start at age 35 for overweight, obese people
New advice from the U.S. Preventive Services Task Force comes as diabetes tech companies look to expand further into the Type 2 population.
By Susan Kelly • Aug. 25, 2021 -
EU builds out MDR, IVDR guidance ahead of flurry of implementing acts
The European Commission has advice on notified bodies, quality management systems and COVID-19 tests, among other topics, as medtechs work to comply with the new and looming regulations.
By Nick Paul Taylor • Aug. 24, 2021 -
Robotic mastectomy's safety and effectiveness unproven, FDA warns
The agency doesn't call out specific firms. Intuitive Surgical is studying its da Vinci Xi system in prophylactic nipple-sparing mastectomy procedures, but tells MedTech Dive it has the required approval.
By Susan Kelly • Updated Aug. 23, 2021 -
Biden said to rule out Woodcock as permanent FDA chief
A published report indicated the agency's longtime drug reviewer is no longer in consideration for the role, leaving it unfilled seven months into President Joe Biden's term.
By Jonathan Gardner • Aug. 20, 2021 -
TAVR sites concentrated in urban areas linked to mortality risk, study finds
Mayo Clinic cardiologists used the findings to call for CMS to withhold reimbursement from sites that fail to meet its original selection or quality criteria.
By Nick Paul Taylor • Aug. 20, 2021 -
EU launches probe of Illumina decision to close Grail deal despite ongoing investigation
If the European Commission finds that Illumina breached a "standstill obligation" rule as part of this latest probe, the company could be fined up to 10% of its revenue.
By Nick Paul Taylor • Updated Aug. 20, 2021 -
Patients were still hesitant to seek medical care out of virus fears this spring, survey finds
Despite reopenings, one in 10 nonelderly adults put off needed medical care this spring, an April survey from the Urban Institute found.
By Hailey Mensik • Aug. 19, 2021 -
Abiomed leads latest FDA breakthrough designations for heart disease devices
The agency awarded the breakthrough privileges to Abiomed's Impella ECP, which the company contends is the world's smallest heart pump and could provide critical hemodynamic support to coronary artery disease patients.
By Nick Paul Taylor • Aug. 19, 2021 -
FDA warns of BlackBerry cyber vulnerability in medical devices
The operating system is often deployed in devices such as cardiac and patient monitors, drug infusion pumps, imaging and surgical robots, according to Nick Yuran, CEO of security consultancy Harbor Labs.
By Greg Slabodkin • Aug. 18, 2021 -
Industry pitches FDA with 26% rise in user fee funding in MDUFA V negotiations
The offer tops $1.2 billion but represents a sharp slowdown in the rate of user fee hikes seen in earlier iterations of MDUFA. AdvaMed declined to comment.
By Nick Paul Taylor • Aug. 18, 2021 -
FDA seeks more power for medical device cybersecurity mandates
CDRH wants to require medtechs to have a Software Bill of Materials ready upfront as part of a premarket submission, as well as the capability to update and patch device security into a product's design.
By Greg Slabodkin • Aug. 17, 2021 -
Boston Scientific's vaginal mesh studies entrench FDA's view that risks outweigh benefits
After halting sales of the product in 2019, the regulator said the additional risks associated with transvaginal mesh repair mean it "continues to believe that these devices do not have a favorable benefit-risk profile."
By Nick Paul Taylor • Aug. 17, 2021 -
Amid delta surge, Thermo Fisher EUA for COVID-19 assays seeks to compensate for variants
FDA in January said accuracy of PCR tests from Thermo Fisher and others may be affected by variants. Testing chief Tim Stenzel said the agency is working to ensure EUA-authorized tests are still performing amid virus mutations.
By Greg Slabodkin • Aug. 16, 2021 -
Abbott's Amulet FDA approval sets stage for US market fight with Boston Scientific
The atrial fibrillation device will challenge Boston Scientific's Watchman in a market valued at $500 million. Abbott plans to release a head-to-head study with its rival at the end of the month.
By Ricky Zipp • Aug. 16, 2021 -
Medtechs need to up their cybersecurity threat modeling game, FDA says
The agency "will be looking for much more detailed and comprehensive" cyber threat models as part of premarket review, said Suzanne Schwartz, director of CDRH's Office of Strategic Partnerships and Technology Innovation.
By Greg Slabodkin • Aug. 13, 2021 -
US now a priority market for most developers of novel devices, FDA survey finds
CDRH topped its goal to boost the percentage of manufacturers that target the U.S. market at least at the same time as rival regions.
By Nick Paul Taylor • Aug. 13, 2021 -
NESTcc seeks feedback on active postmarket medical device surveillance system
The National Evaluation System for health Technology plans to work with a contractor to test real world scenarios for devices such as ventilators, robotic surgery, breast implants and fully disposable duodenoscopes.
By Nick Paul Taylor • Aug. 13, 2021 -
Boston Scientific gets FDA nod for single-use bronchoscope, sees $2B opportunity
The medtech giant is targeting a potentially lucrative market but will face competition from rivals with a longer history in the space, including Ambu and Olympus. A limited U.S. release of the device is set for the coming weeks.
By Nick Paul Taylor • Aug. 11, 2021 -
'Evidence gaps' spur CMS to call meeting to discuss cerebrovascular devices
The agency said the "shorter term data with greater reliance upon intermediate and surrogate outcomes" used to bring devices to market is "generally less helpful" for its assessments.
By Nick Paul Taylor • Aug. 11, 2021 -
Opinion
Digital therapeutics — a double-edged sword?
Aloha McBride, EY's global health leader, argues that algorithms need to be regularly audited and screened for bias and discrepancies, while clinicians play a vital role in explaining the key features of apps and patient privacy risks.
By Aloha McBride • Aug. 10, 2021 -
Delaware tries capping hospital price growth to fund more primary care
A multi-pronged bill also forces certain payers to tie nearly half of their business to alternative payment models by 2023, and create shared accountability for both the cost and quality of care.
By Samantha Liss • Aug. 9, 2021 -
5 must-watch panels at an unprecedented HIMSS21
Masks and proof of vaccination will be required on the ground in Las Vegas, and a corresponding digital event will also take place.
By Rebecca Pifer Parduhn • Aug. 9, 2021










