FDA: Page 68
-
FDA Breakthrough Devices Program nears 300 designations
The agency has tapped 50 devices so far this year, with recent nods to Orteq, Thermedical, ArcherDX, Helius, PhotoPharmics and Terumo.
By Susan Kelly • May 27, 2020 -
CDC seeks to limit false positives with COVID-19 antibody testing, as immunity question lingers
The interim guidelines promote using high-specificity assays and conducting confirmatory tests when appropriate. However, the agency won't yet use serologic test results to sway public health recommendations.
By Nick Paul Taylor • May 27, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA eases rules on changes to PMA, HDE devices to avoid coronavirus supply disruptions
Companies that market medical devices under premarket approvals or humanitarian device exemptions can make limited modifications to design and production without giving prior notice, according to special guidance.
By Nick Paul Taylor • May 22, 2020 -
Abbott, Roche ink deals with UK for 10M coronavirus antibody tests
The notice from the government, which bought $20 million in unreliable antibody tests early in the crisis, came as FDA removed 28 products from a list of the tests that can be used without emergency use authorizations.
By Nick Paul Taylor • May 22, 2020 -
Hospitals push FEMA to form broad supply pact with medtech
The agency on Thursday is set to discuss a proposed five-year voluntary agreement with industry to better distribute medical products, which health systems hope will go beyond personal protective equipment.
By Nick Paul Taylor • Updated May 26, 2020 -
COVID-19 drives EC to change rules on notified body designations
The European Commission is allowing certain deviations from normal procedures governing notified bodies, enabling renewal of designations under the outgoing device regulations without performing on-site assessments.
By Nick Paul Taylor • May 20, 2020 -
Labs welcome CMS rate for coronavirus antibody testing
Analysts at William Blair said the roughly $42 rate for common serological tests is higher than expected, which may bode well for antigen testing reimbursement.
By Nick Paul Taylor • May 20, 2020 -
FDA OKs 1st at-home collection kit for use with multiple labs, coronavirus tests
The emergency use authorization to Everlywell follows earlier nods to LabCorp and Rutgers Clinical Genomics Laboratory for at-home specimen collection for analysis solely at their own labs.
By Nick Paul Taylor • May 18, 2020 -
Allergan's breast implant postmarket studies 'unacceptable,' FDA warns
Within the last 14 months, every manufacturer of the controversial devices allowed for sale in the U.S. has received a warning letter from the agency.
By Maria Rachal • May 15, 2020 -
FDA revokes umbrella EUA for infusion pumps due to lack of industry use
The agency told medtechs it may instead grant individual EUAs for the devices going forward. B. Braun contends it is the only company with such an emergency authorization and is not affected by the revocation.
By Greg Slabodkin • Updated Sept. 24, 2020 -
Cardiology group ranks best devices for aorto-iliac arterial interventions
The Society for Cardiovascular Angiography and Interventions detailed stances on several key issues, including endovascular device effectiveness, issued Thursday at its virtual conference.
By Nick Paul Taylor • May 15, 2020 -
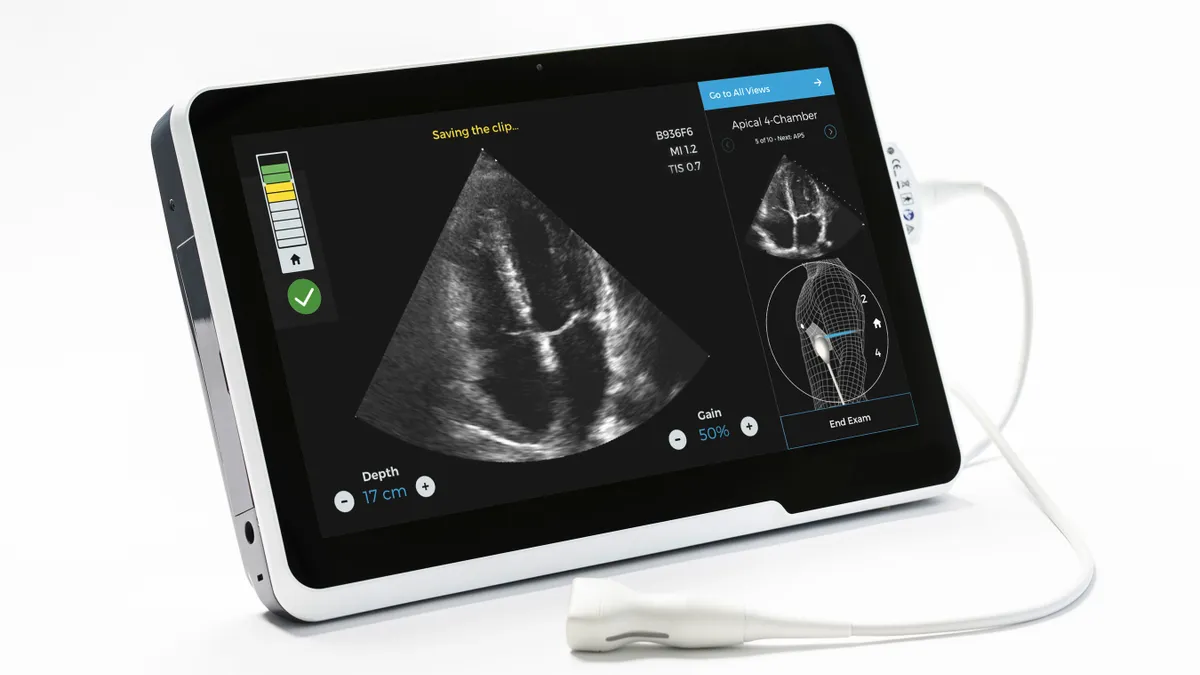
Philips, startups look to deploy new ultrasound tech amid coronavirus
The need for point-of-care imaging for lung and cardiac complications among COVID-19 patients has led to a flurry of expedited FDA reviews.
By Nick Paul Taylor • May 14, 2020 -
EC outlines clinical trial safety reporting between MDR, Eudamed start dates
Even with the recent delay to the date of application of the Medical Device Regulation, there will still be at least a 12-month period when the key Eudamed database is not fully available.
By Nick Paul Taylor • May 14, 2020 -
House Dems carve out $75B for coronavirus testing, contact tracing in bid for new relief package
The $3 trillion bill passed the House by a vote of 208-199 on Friday. It's Democrats' opening gambit for the next wave of congressional action, but Republicans say they're not in a hurry to approve new funding.
By Susan Kelly • Updated May 18, 2020 -
FDA flags 2nd high-risk recall of embolectomy catheters in recent weeks
The potential for a detached catheter tip to damage blood vessels and cause serious injuries or death underpinned the agency’s Class I label for an Applied Medical recall on Tuesday, seven months after the company initiated the recall.
By Nick Paul Taylor • May 13, 2020 -
Eko algorithm gets FDA nod for cardiac screening of COVID-19 patients
Developed with the Mayo Clinic, Eko's algorithm gained breakthrough device status in December and review was further accelerated given its potential to identify patients at higher risk of poor outcomes with the coronavirus.
By Nick Paul Taylor • May 13, 2020 -
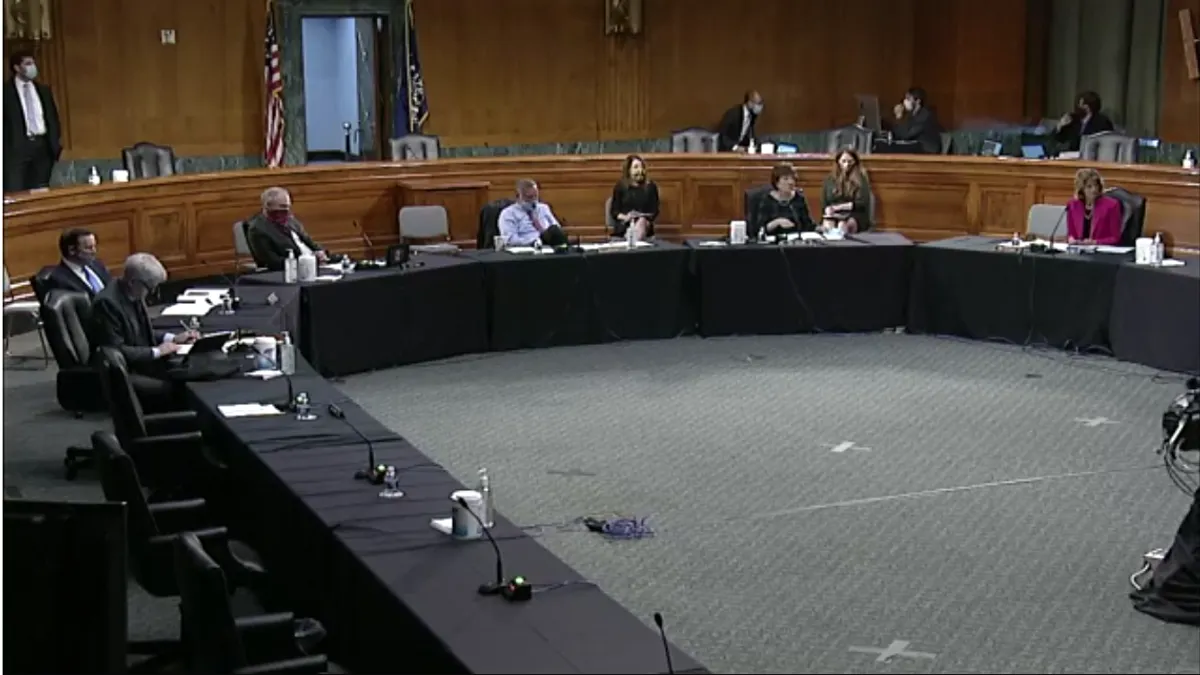
As Trump pushes states to reopen, Fauci warns against lifting COVID-19 restrictions too soon
The U.S. doesn't have the necessary testing and surveillance infrastructure in place to prep for a fall resurgence of the coronavirus, a second wave that's "entirely conceivable and possible," Fauci told a Senate panel.
By Rebecca Pifer Parduhn • May 12, 2020 -
Abiomed faces familiar reimbursement fluctuation in CMS proposal
An almost 25% reduction to a key heart implant procedure payment code, plus backing for three breakthrough-designated devices, are among the policies outlined in a Medicare proposed rule released Monday afternoon.
By Maria Rachal • May 12, 2020 -
LabCorp at-home collection test kit for coronavirus goes mainstream, as states get $11B for testing efforts
The tests, originally only offered to frontline workers, are now available even to individuals without symptoms who may have been exposed to the virus. Still, clinical labs say more support is needed to further ramp up capacity.
By Greg Slabodkin • May 12, 2020 -
FDA, CDC drawing up plan to restart routine facility inspections
A phased approach is in the works to reintroduce certain oversight that's been on hold both domestically and internationally since the coronavirus outbreak reached pandemic level in March.
By Nick Paul Taylor • May 12, 2020 -
Latest Abbott coronavirus antibody test receives FDA emergency use OK
The EUA is the company's second for a test detecting the IgG antibody, using a different machine than the first. Across both, it plans to ship almost 30 million units worldwide in May and double that amount in June.
By Greg Slabodkin • May 11, 2020 -
1st antigen test to detect coronavirus gets FDA nod
The agency, which gave the green light on Saturday to Quidel, expects the tests to be cheaper to make than PCR tests.
By Nick Paul Taylor • May 11, 2020 -
FDA details COVID-19 era reporting rules to stymie medical device shortages
Under new powers granted by the CARES Act for the duration of the health crisis, the guidance to industry lays out when and how to notify the agency about changes that could affect product availability.
By Nick Paul Taylor • May 7, 2020 -
Notified bodies saw CE marks spike in 2019 ahead of now-delayed MDR
Team-NB attributed the surge to an industry push to get certified under the outgoing Medical Device Directive, before the COVID-19 pandemic led the EU to postpone the new regulations start date by a year.
By Nick Paul Taylor • May 6, 2020 -
FDA beefs up coronavirus antibody test regs critics called 'recipe for disaster'
The Association of Public Health Laboratories praised the move. "This revised policy makes a lot of sense and should have been in place over the last six weeks," the group's CEO Scott Becker said.
By Greg Slabodkin • May 4, 2020