Medical Devices: Page 114
-
Diabetes tech companies continue 2020's success with Q1 growth. Investors seem unimpressed.
Dexcom, Insulet and Tandem all grew revenue or sales in Q1, winning over Wall Street analysts but seemingly not investors. Meanwhile, the diabetes tech space grows with FDA's clearance of Bigfoot Biomedical's smart insulin pen.
By Ricky Zipp • May 12, 2021 -
EU plans to impose additional regulations on medtech AI products, other 'high-risk' systems
The proposed legal framework, which addresses potential artificial intelligence risks, seeks to regulate the technology and issue fines for noncompliance that could total billions of dollars, positioning Europe for a leading AI role globally.
By Nick Paul Taylor • May 12, 2021 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medtronic's HVAD controversy
Medtronic hit with another Class I recall regarding HeartWare HVAD system
The recall for instructions and patient manuals marks the system's third Class I recall in 2021 and fifth since 2018. One death and 64 injuries have been reported due to issues from the most recent recall.
By Ricky Zipp • May 12, 2021 -
OIG calls on EPA to review ethylene oxide cancer risks, possibly delaying new regulations until 2022
The Office of Inspector General wants the agency to conduct a fresh review of cancer risks from EtO emissions associated with medical device sterilization facilities. EPA's final commercial sterilizer rule had been slated for 2021.
By Susan Kelly • May 11, 2021 -
FTC asks Allergan Aesthetics, Soliton for additional information on proposed $550M buyout
The regulator's second request for documentary material, which comes three months after the proposed deal was announced, is part of a deeper probe than its standard review of M&A.
By Nick Paul Taylor • Updated Aug. 11, 2021 -
CMS implementation of breakthrough payment rule may be further delayed: Cowen
The agency will most likely push the start date back further to address the concerns of critics of the contentious Medicare Coverage of Innovative Technology initiative, an analyst contends.
By Nick Paul Taylor • May 10, 2021 -
TAVR readmission rates vary widely between hospitals for unknown reasons: study
Length of stay and discharge disposition only explained 15% of the inter-hospital variation. The study's authors have called for efforts to identify practices associated with low rates or readmission.
By Nick Paul Taylor • May 10, 2021 -
Robotic surgery outlook boosted by hospital spending pickup in Q1
Intuitive and Stryker said that system buys were stronger than expected in the first quarter. Zimmer also reported momentum for its Rosa system, while Globus had positive early feedback for its robotic spine technology.
By Susan Kelly • May 10, 2021 -
With MDR nearing, EU updates guidance on transition to Eudamed database
The European Union no longer requires reporting obligations to be carried out as soon as the database becomes fully functional. The Medical Device Regulation takes effect May 26 and Eudamed will come online one year later.
By Nick Paul Taylor • May 7, 2021 -
iRhythm again meets with Medicare rate setter after cardiac monitoring pay cut by nearly $200
CEO Michael Coyle said during a Thursday earnings call the company has met with Novitas Solutions to discuss a different pricing methodology. Investors seemed encouraged as shares were up nearly 8% Friday morning.
By Ricky Zipp • May 7, 2021 -
FDA shares plan for restarting facility inspections, warns backlog will persist into 2022
In a projected base-case scenario, the agency expects to have cleared 26% of its backlog of medical product inspections by October. A total of 2,426 device and radiological health inspections remain for fiscal year 2021.
By Nick Paul Taylor • May 7, 2021 -
Nevro raises outlook as neuromodulation market recovers from COVID-19 crisis
Improvements during the quarter, which culminated in Nevro's best March ever, emboldened the company to target at least 22% growth in 2021. Still, the company's stock price dropped by about 6% Thursday morning.
By Nick Paul Taylor • May 6, 2021 -
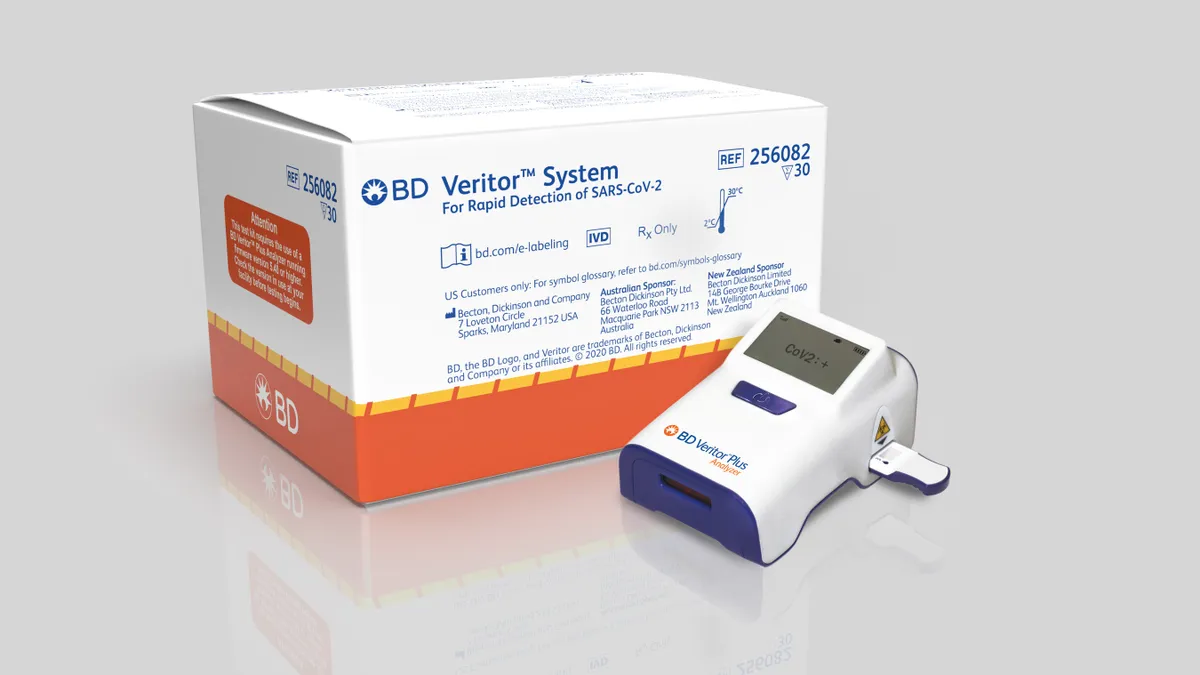
BD grows Q2 revenue despite COVID-19 test dip, spins off diabetes business
The spin off for the separate publicly traded company is expected to be completed in the first half of calendar year 2022. The move will not impact BD's ability to fulfill orders related to COVID-19 vaccinations, execs said.
By Greg Slabodkin • May 6, 2021 -
AI, digital health feature in latest batch of FDA breakthrough device designations
The agency granted regulatory privileges to artificial intelligence products, including software designed to enable the early detection of cancer and technology to help surgeons tell if cancer is still present after surgical excision.
By Nick Paul Taylor • May 5, 2021 -
Newer TAVR devices linked to lower rate of permanent pacemaker use: study
The 1,987-subject study found patients receiving older transcatheter heart valves, such as Edwards Lifesciences' Sapien and Medtronic's CoreValve, required permanent pacemakers at a higher rate than newer-generation THVs.
By Nick Paul Taylor • May 5, 2021 -
Zimmer sees Q1 sales growth after CEO warned of down quarter
Bryan Hanson cautioned in February the quarter would be more challenging than the fourth due to COVID-19 pressures. The turnaround led the company to issue full-year 2021 guidance, but investors weren't impressed.
By Ricky Zipp • May 4, 2021 -
Q&A
Radiology Partners, Aidoc talk AI adoption, handling bias, FDA actions
While AI and machine learning technologies have gained popularity in the medtech and healthcare industries, the companies intend to further grow usage in radiology with their recent partnership.
By Ricky Zipp • May 3, 2021 -
Orthopaedic companies project 'return to normalcy' as electives recover in Q1
J&J, Smith & Nephew and Stryker reported seeing a light at the end of the tunnel during the first quarter as nonessential care rebounded, with a backlog of hip procedures pointing the way forward.
By Susan Kelly • May 3, 2021 -
CMS finalizes joint replacement pricing extension with input from medtech industry
Cowen analysts say the agency's initiative could evolve in ways that put pressure on the prices of orthopaedic devices. However, they contend companies can mitigate that threat by selling more products to customers.
By Nick Paul Taylor • April 30, 2021 -
Smith & Nephew returns to growth as US hip sales surge
First quarter orthopaedic results saw divergent performance in the knees and hip franchises. CEO Roland Diggelmann noted hip injuries are typically more acute but acknowledged a gap in its U.S. knee portfolio.
By Nick Paul Taylor • April 29, 2021 -
Stryker's Wright costs spur Q1 miss, but robotics orders pick up
The medtech also raised its full-year earnings outlook, which CFO Glenn Boehnlein said is based on an assumption that the second quarter "has a return to normalcy by the time we get to the end."
By Susan Kelly • April 28, 2021 -
Boston Scientific sees Q1 electives rebound, hints at further 2021 M&A
With two acquisitions already in 2021, CFO Daniel Brennan said, "We have the appetite, and we have the balance sheet in place to continue to" spend later this year.
By Ricky Zipp • April 28, 2021 -
CMS pitches extra year of add-on payments for Boston Scientific, Stryker, other devices
Boston Scientific's Eluvia drug-eluting stent, Cook Medical's Hemospray and Stryker's SpineJack system are among those that would get the extension under the proposed fiscal 2022 inpatient hospital rule.
By Nick Paul Taylor • April 28, 2021 -
GE Healthcare grows sales as routine screening, elective procedures return
The results provide further evidence that the resumption of elective procedures has driven up demand for imaging and other healthcare products and services. GE saw volumes return for cardiac, oncology and neurology screening.
By Nick Paul Taylor • April 27, 2021 -
Philips raises guidance as elective procedures exceed pre-pandemic levels
The Dutch conglomerate's results follow encouraging rebounds in non-emergency care from Edwards, Intuitive Surgical and J&J. Still, Philips posted a loss from continuing operations.
By Nick Paul Taylor • April 26, 2021