Medical Devices: Page 30
-
Stryker, Zimmer outline different M&A strategies
Stryker CEO Kevin Lobo told investors M&A will be the “number one use of our cash going forward,” while Zimmer Biomet CEO Ivan Tornos emphasized internal product development for growth.
By Elise Reuter • Oct. 30, 2024 -
Exactech files for Chapter 11, agrees to investor buyout
The filing follows Exactech’s failure to settle product liability claims from about 2,600 patients in response to recalls of orthopedic devices.
By Nick Paul Taylor • Oct. 30, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
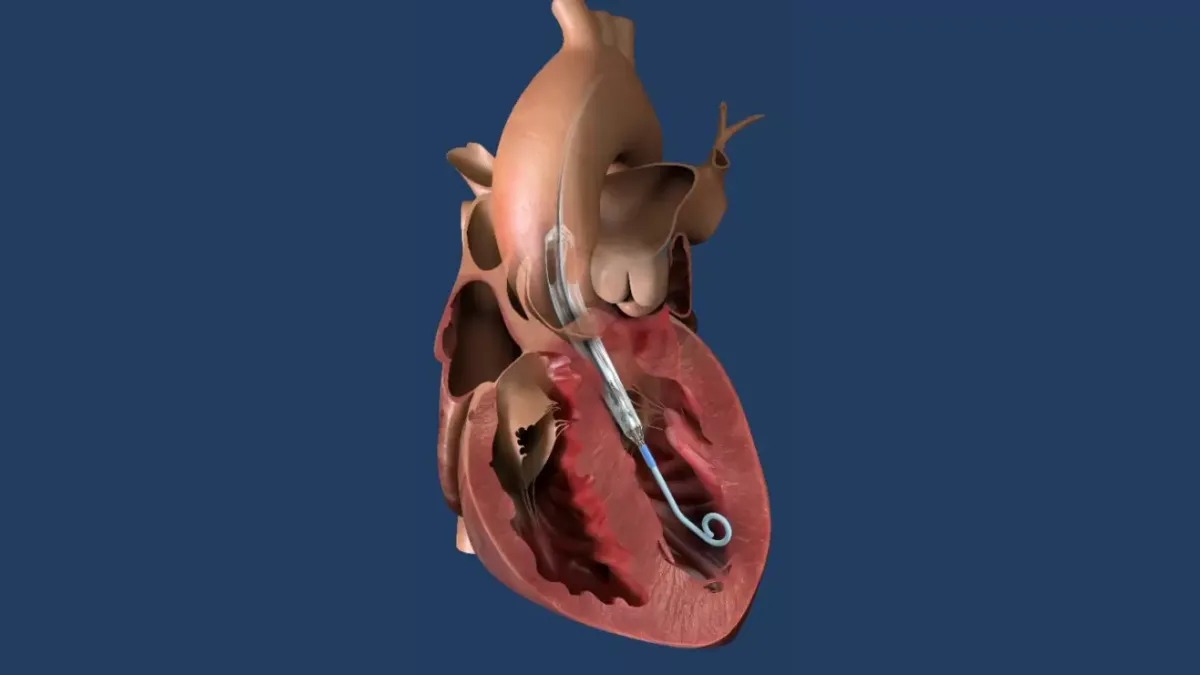
Edwards data show benefit of early TAVR over ‘watchful waiting’
Study author Philippe Genereux said the trial data “shatter 60 years of ingrained belief on the treatment for severe aortic stenosis.”
By Susan Kelly • Oct. 29, 2024 -
J&J to seek FDA approval after small-bore Impella heart pump hits trial goal
The company has predicted the narrower Impella ECP will be easier to insert and implant, as well as enable the use of small bore access and closure techniques.
By Nick Paul Taylor • Oct. 29, 2024 -
Boston Scientific to close Silk Road Medical headquarters, lay off 138 people
Following its acquisition of Silk Road in September, Boston Scientific said it plans to consolidate the company’s work in Minnesota.
By Elise Reuter • Oct. 28, 2024 -
Philips cuts sales forecast after ‘significant deterioration’ in China
CEO Roy Jakobs expects the slump to continue into 2025, driven by China’s anti-corruption measures.
By Nick Paul Taylor • Oct. 28, 2024 -

 Retrieved from Hologic on May 23, 2024
Retrieved from Hologic on May 23, 2024
FDA advises clinicians to stop using Hologic radiographic markers
Hologic has recalled the Biozorb devices after 188 reports of adverse events in patients who received the implants in breast tissue.
By Susan Kelly • Oct. 28, 2024 -
Dexcom chief commercial officer to retire
Teri Lawver is leaving the diabetes tech company during a challenging time, with sales slowing after a workforce restructuring.
By Elise Reuter • Oct. 25, 2024 -
Edwards’ TAVR sales faced ongoing hospital constraints in Q3
Hurt by a slowdown in procedures for its heart valve replacement devices, Edwards said it is working with hospitals to manage workflow challenges.
By Susan Kelly • Oct. 25, 2024 -
Baxter to restart high-throughput IV solutions manufacturing line
Baxter still does not have a timeline for fully restoring production at the North Carolina facility, which was hit by Hurricane Helene.
By Elise Reuter • Oct. 25, 2024 -
Medtronic wins FDA approval for Affera mapping and ablation system
Affera combines mapping technology with a catheter capable of performing radiofrequency and pulsed field ablation.
By Nick Paul Taylor • Oct. 25, 2024 -
European Parliament calls for proposals to reform MDR and IVDR by end of Q1
Parliament asked the European Commission to publish acts that address the “most pressing challenges and bottlenecks” of the regulations and propose the “systematic revision” of the legislation.
By Nick Paul Taylor • Oct. 24, 2024 -
The Medtech Conference
Medtech Conference recap: New CDRH leader details approach; AI and LDTs in focus
Catch up on our recent coverage of Advamed’s The Medtech Conference.
By Elise Reuter • Oct. 23, 2024 -

 Retrieved from iRhythm on October 22, 2024
Retrieved from iRhythm on October 22, 2024
iRhythm’s Zio AT design changes win FDA clearance
The agency cleared one of two 510(k) submissions iRhythm filed for the heart monitor after receiving a warning letter from the agency last year.
By Susan Kelly • Oct. 23, 2024 -
Boston Scientific boosts PFA expectations, citing rapid Farapulse adoption
Demand for the pulsed field ablation device drove the company’s electrophysiology sales up 177% in the third quarter compared to a year ago.
By Susan Kelly • Oct. 23, 2024 -
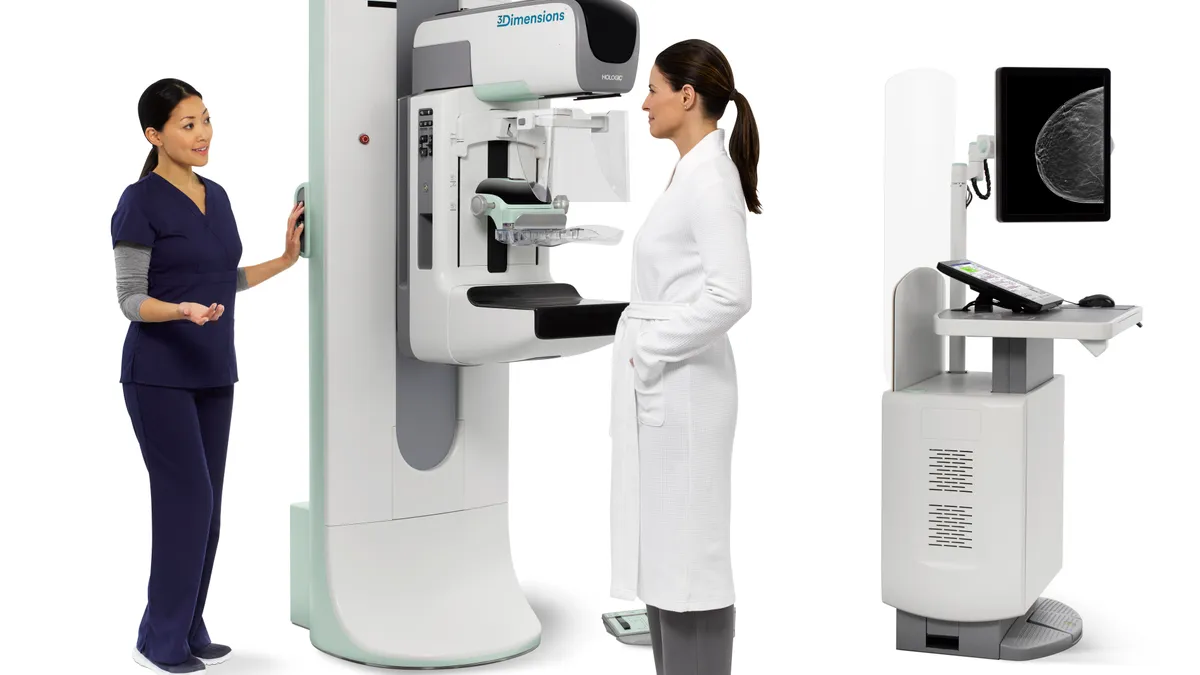
Hologic to lay off 86 people in closure of Connecticut facility
The company committed to closing the facility, which makes breast health products, in late 2021 and gave most employees the option to relocate to other sites.
By Nick Paul Taylor • Oct. 23, 2024 -
Tarver named new director of FDA’s device center
Michelle Tarver, who will officially replace longtime CDRH leader Jeff Shuren, emphasized the agency’s focus on patients in comments on Oct. 17.
By Elise Reuter • Oct. 22, 2024 -
The Medtech Conference
That’s a wrap: 5 takeaways from The Medtech Conference
The conference — Advamed’s largest — featured an appearance by the device center’s new acting director and sessions on AI, clinical trial diversity and the FDA’s contentious LDT rule.
By Elise Reuter • Oct. 22, 2024 -
Boston Scientific, Edwards and Dexcom usher in second week of earnings
Johnson & Johnson, Abbott and Intuitive Surgical made a mixed start to earnings season last week.
By Nick Paul Taylor • Oct. 22, 2024 -
Boston Scientific blood-blocking agent tied to additional 2 deaths, 8 injuries
After a February recall, Boston Scientific has warned physicians about new safety risks for Obsidio Embolic, which is now connected to a total of 15 injuries and four deaths.
By Nick Paul Taylor • Oct. 21, 2024 -
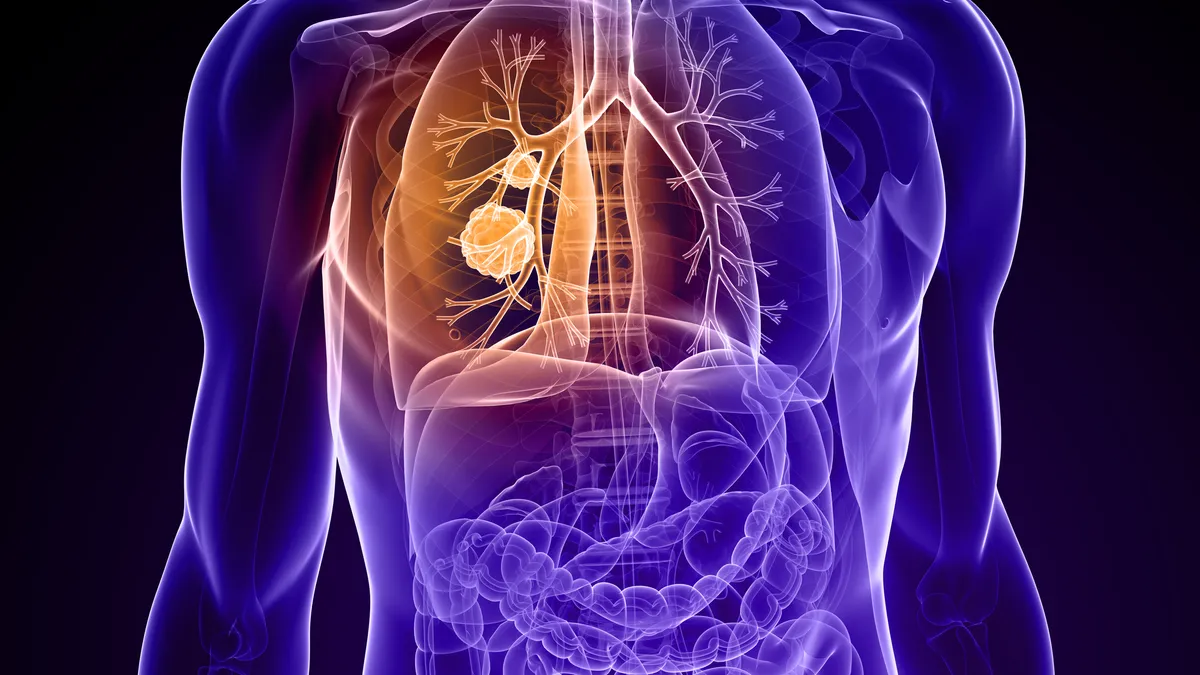
Novocure wins FDA approval for electric field device in lung cancer
Alongside immunotherapy or chemo, the wearable device helped patients live longer in a trial, though the results are somewhat controversial.
By Jonathan Gardner • Oct. 18, 2024 -
The Medtech Conference
Tarver, acting CDRH director, sets tone for future of the device center
Former CDRH leader Jeff Shuren told an audience at The Medtech Conference that Michelle Tarver “will make an excellent center director, and quite frankly, the best is yet to come.”
By Elise Reuter • Oct. 18, 2024 -
Intuitive readies da Vinci 5 for broader launch after placing 110 robots in Q3
BTIG analysts wrote that installations of Intuitive’s new robotic surgery system are “well ahead of lofty” expectations.
By Susan Kelly • Oct. 18, 2024 -
FDA prioritizes guidance on lab developed tests in 2025 plan
The Center for Devices and Radiological Health intends to develop final guidance on its enforcement discretion policy regarding special controls for LDTs, among other topics.
By Nick Paul Taylor • Oct. 18, 2024 -
Medtech Europe calls for urgent reform of MDR and IVDR
The trade group’s intervention follows news that the new commissioner for Health and Animal Welfare will assess the need for legislative changes.
By Nick Paul Taylor • Oct. 18, 2024