Medical Devices: Page 29
-
Trump nominates RFK Jr. to lead HHS
The nomination of a prominent vaccine skeptic to the head of the nation’s largest healthcare program is expected to alarm some public health experts.
By Sydney Halleman • Nov. 14, 2024 -
FDA breakthrough device decisions rebound after recent declines
The FDA designated 166 products under its breakthrough devices program in its most recent financial year, reversing the downward trend seen as the COVID-19 pandemic eased.
By Nick Paul Taylor • Nov. 14, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
 Retrieved from GE Healthcare on November 14, 2024
Retrieved from GE Healthcare on November 14, 2024
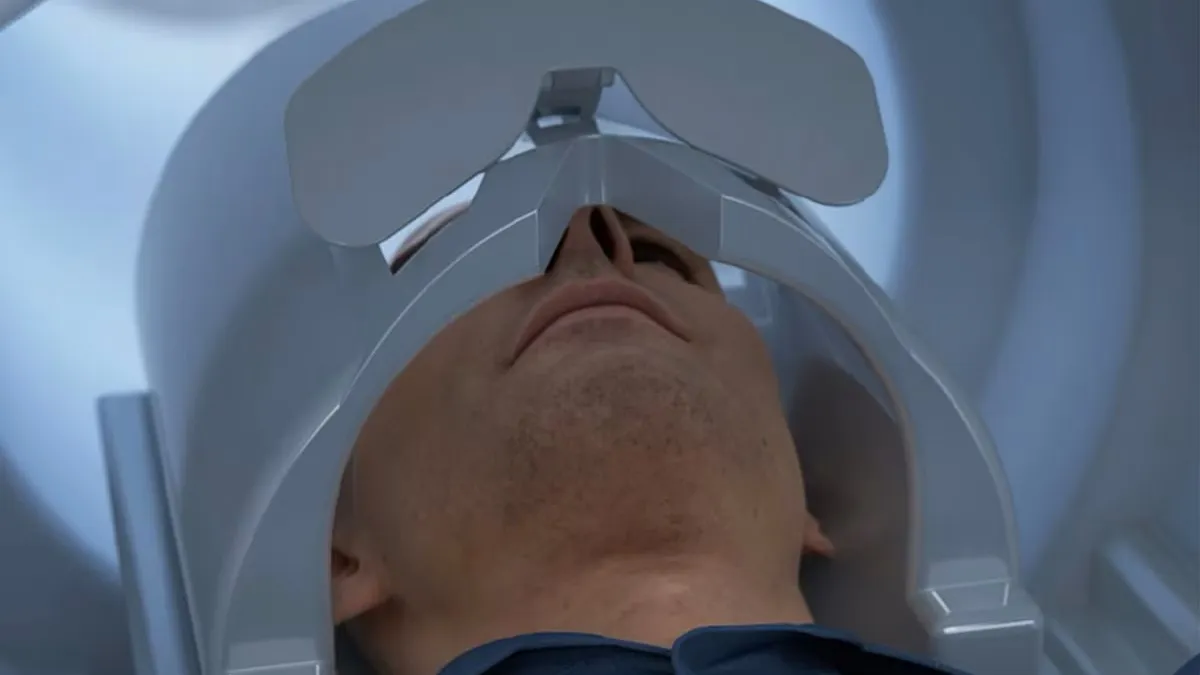
GE Healthcare’s head-only MRI scanner cleared by the FDA
The system is designed to shorten scan times, which may be better for people who struggle to stay still or have claustrophobia, and to detect more subtle abnormalities.
By Nick Paul Taylor • Nov. 14, 2024 -
Haemonetics to close California facility, lay off 75 people
Over the past year, Haemonetics has reshaped its product portfolio through restructuring and M&A, adding devices used in cardiology procedures.
By Susan Kelly • Nov. 14, 2024 -
Dean Kamen, insulin pump pioneer, wants to shake up diabetes tech
Kamen and Sequel Med Tech CEO Alan Lotvin say they are offering greater precision in insulin delivery with new pump technology in the Twiist device.
By Elise Reuter • Updated Nov. 25, 2024 -


Photo by MART PRODUCTION from Pexels

Livanova hits sleep apnea trial goals, plans FDA filing
Leerink analysts said the results were largely in line with outcomes in a trial of Inspire Medical’s rival device but cautioned that Livanova may have a hard time breaking into the sleep apnea market.
By Nick Paul Taylor • Nov. 13, 2024 -
J&J’s Ottava surgical robot to begin clinical trial after FDA nod
With FDA approval of an investigational device exemption, J&J’s soft tissue robot moves a step closer to challenging Intuitive Surgical’s da Vinci system.
By Susan Kelly • Nov. 12, 2024 -
Boston Scientific resumes PFA trial in new patient group after pausing enrollment
Boston Scientific expects to complete enrollment in the coming months in the study comparing pulsed field ablation to anti-arrhythmic drugs in patients with persistent AFib, a spokesperson said.
By Susan Kelly • Nov. 12, 2024 -
Advamed CEO congratulates Trump, stresses need for public policy support
Donald Trump’s election victory comes weeks after the FDA named a new leader for the medical device center and as the agency’s contentious LDT final rule is challenged in court.
By Ricky Zipp • Nov. 11, 2024 -
How the healthcare industry is reacting to a second Trump term
Donald Trump’s first term as president was characterized by significant turbulence for government healthcare programs. Here’s how some of the most influential industry groups responded to the Republican’s reelection.
By Rebecca Pifer Parduhn • Nov. 11, 2024 -
Inquis raises $40M for pivotal trial of clot removal device
The Aventus thrombectomy system uses technology that tells operators if the device’s tip is in contact with blood, a clot or vessel walls.
By Nick Paul Taylor • Nov. 7, 2024 -
Siemens Healthineers confident it can cope with Trump tariffs
CEO Bernd Montag said the company is well positioned for a “U.S. versus China situation” that could arise when Donald Trump returns to the White House.
By Nick Paul Taylor • Nov. 7, 2024 -
Masimo, amid leadership transition, targets cost reductions
Executives said the patient monitoring company’s consumer business will likely become a discontinued operation if it is not spun off.
By Susan Kelly • Nov. 6, 2024 -
Tempus AI to buy genetic testing company for $600M
CEO Eric Lefkofsky said the purchase of Ambry Genetics will expand Tempus’ testing portfolio and bring in a fast-growing business.
By Elise Reuter • Nov. 6, 2024 -
Advamed asks CMS to cover extra imaging of dense breast tissue
Many patients with dense breast tissue currently have to pay out of pocket or forgo potentially life-saving additional testing, Advamed said in a letter.
By Nick Paul Taylor • Nov. 6, 2024 -
Medtronic, Recor win CMS transitional payment coverage for renal denervation devices
Patient and physician education is the next challenge to change the treatment paradigm for drug-resistant hypertension, Citi Research analyst Joanne Wuensch said.
By Susan Kelly • Nov. 5, 2024 -
Integra Lifesciences names Mojdeh Poul as CEO
Poul will take over the company in January after spending 11 years with 3M and working at Medtronic and Boston Scientific.
By Nick Paul Taylor • Nov. 5, 2024 -
Boston Scientific continues M&A run with Cortex buy
Cortex adds to Boston Scientific’s electrophysiology portfolio amid treatment shifts in the space driven by new pulsed field ablation devices.
By Ricky Zipp • Nov. 4, 2024 -
Medtronic settles pulse oximetry lawsuit alleging inaccuracies
Roots Community Health has sued 12 other firms, including GE Healthcare, CVS and Walgreens, and called for manufacturers and the FDA to ensure the devices work for everyone.
By Elise Reuter • Nov. 4, 2024 -
Zimmer licenses NeuroOne brain ablation system
NeuroOne will get $3 million upfront for an exclusive license to distribute its radiofrequency ablation system designed to treat neurological disorders such as epilepsy and Parkinson's disease.
By Nick Paul Taylor • Nov. 4, 2024 -
J&J Medtech CIO Larry Jones resigns
Jones, who has led connectivity solutions for J&J’s surgical robots, announced his departure on LinkedIn.
By Elise Reuter • Updated Nov. 1, 2024 -

 Retrieved from iRhythm on October 22, 2024
Retrieved from iRhythm on October 22, 2024
FDA clears iRhythm’s second 510(k) in response to warning letter
However, the company said it will delay a submission for its next-generation Zio cardiac monitoring system until remediation efforts are further along.
By Susan Kelly • Nov. 1, 2024 -
EU posts guidance on ethylene oxide in device sterilization
The document clarifies that EtO falls within the scope of the Medical Device Regulation and the In Vitro Diagnostic Regulation.
By Nick Paul Taylor • Nov. 1, 2024 -
Edwards, Medtronic and Inari share trial data at TCT conference
The three medtech firms unveiled new data this week during TCT 2024 in Washington, D.C. Here’s what the results mean for the companies and their device markets.
By Nick Paul Taylor • Oct. 31, 2024 -
Boston Scientific’s Acurate Neo2 inferior to rival TAVR valves in study
Analysts said the findings complicate the outlook for a U.S. launch of Boston Scientific's aortic valve replacement device.
By Susan Kelly • Oct. 31, 2024