Medical Devices: Page 4
-
FDA needs more staff, authority to oversee device recalls, watchdog finds
“FDA's challenges — such as insufficient staffing — can create inefficiencies in the process and potentially put lives at risk,” the U.S. Government Accountability Office said.
By Elise Reuter • Dec. 15, 2025 -
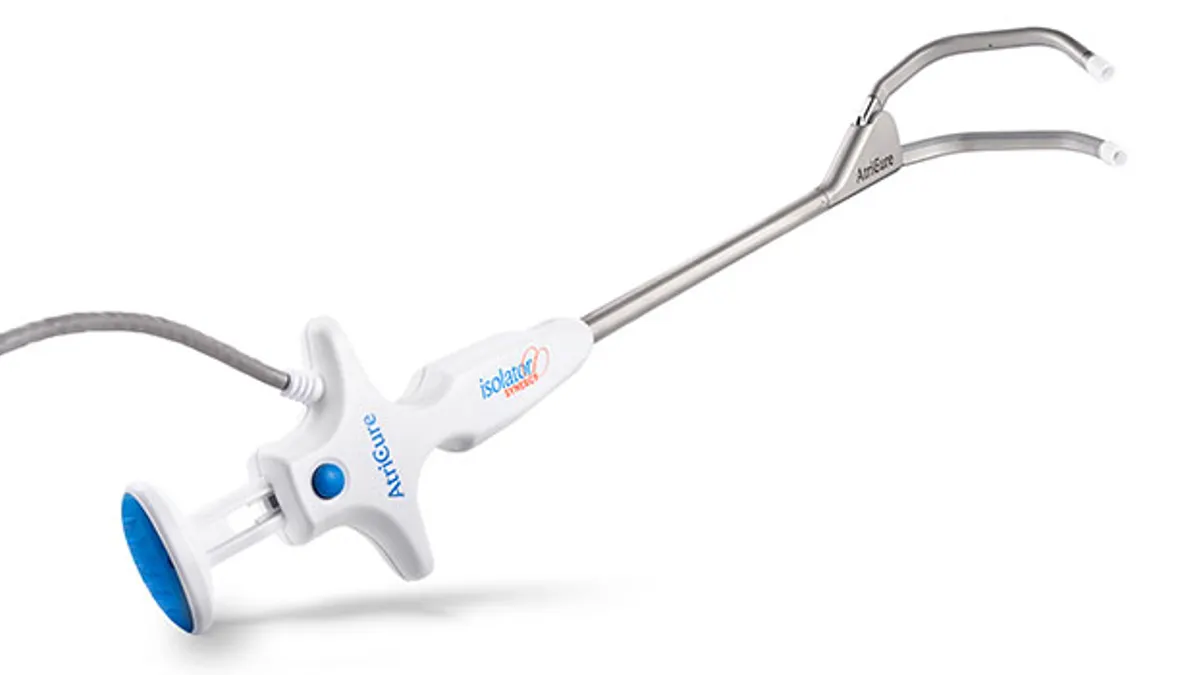
 Retrieved from AtriCure on December 12, 2025
Retrieved from AtriCure on December 12, 2025
AtriCure treats first patients with dual PFA/RF system
Combining pulsed field ablation and a radiofrequency technique in one platform is expected to reduce procedure times.
By Susan Kelly • Dec. 12, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Mick Farrell named chair of AdvaMed board
The Resmed CEO will serve a two-year term on the medtech lobbying group’s board.
By Elise Reuter • Dec. 12, 2025 -
Impulse Dynamics raises $158M to commercialize heart failure device
By strengthening heart contractions, Impulse’s device could improve quality of life, restore functional capacity and enhance tolerance for exercise.
By Nick Paul Taylor • Dec. 11, 2025 -
Intuitive wins 3 new indications for da Vinci SP system
The robotic surgery market leader’s single port system can now be used in inguinal hernia repair, gallbladder removal and appendectomy procedures.
By Susan Kelly • Dec. 11, 2025 -
FDA pilots allowing digital health devices access to CMS payment program
Companies can ask the FDA to waive premarket authorization and investigational device requirements while they collect real-world data in a CMS program.
By Nick Paul Taylor • Dec. 10, 2025 -
Teleflex inks deals to sell 3 businesses for $2B
Needham analysts said Teleflex’s inability to secure a price above the low end of their expectations could reflect “the continued poor performance of UroLift.”
By Nick Paul Taylor • Dec. 10, 2025 -
GE HealthCare, Mayo Clinic expand partnership around radiology research
Ben Newton, GE HealthCare’s global head of oncology, said the partnership will shift radiation therapy from a “one-size-fits-all” approach to a personalized method.
By Elise Reuter • Dec. 9, 2025 -
Medtronic changes investor relations leadership ahead of diabetes spinout
Ryan Weispfenning has elected to take up the challenge of creating and leading the investor relations function at the planned new diabetes company.
By Nick Paul Taylor • Dec. 9, 2025 -
Deep Dive
What medtech firms can learn from Whoop’s warning letter
An FDA warning letter about the wearable company’s blood pressure feature raises questions about the blurring lines between medtech and wellness.
By Elise Reuter • Dec. 8, 2025 -
SS Innovations submits robotic system for FDA clearance
After introducing its robot in multiple countries outside the U.S., including India, the company turns its focus to the world’s largest robotic surgery market.
By Susan Kelly • Dec. 8, 2025 -
Olympus-backed Swan EndoSurgical names Erik Todd as CEO
Swan highlighted Todd’s experience in scaling robotic platforms, building global engineering organizations and driving innovation across endoscopy and minimally invasive surgery.
By Nick Paul Taylor • Dec. 5, 2025 -
FDA advisory panel votes unanimously against J&J heart shunt
Panelists focused on study data that showed the implant did not improve outcomes in heart failure patients.
By Susan Kelly • Dec. 5, 2025 -
Stryker names Spencer Stiles as COO
Stiles has worked at Stryker for 27 years, most recently leading the company’s orthopedics business.
By Elise Reuter • Dec. 4, 2025 -
Olympus reinforces advice over device issue linked to 113 serious injuries
The company received complaints that the ligation loop can become unintentionally anchored in place.
By Nick Paul Taylor • Dec. 4, 2025 -
Medtronic’s Hugo surgical robot earns FDA clearance
With the U.S. authorization for urologic procedures, “there is now choice” for hospitals that want to expand their robotic programs, a company executive said.
By Susan Kelly • Dec. 4, 2025 -
Medtronic launches insulin delivery system with Abbott-made sensor
The partnership is the first time Medtronic has integrated its insulin pumps with a sensor developed outside of the company.
By Elise Reuter • Dec. 3, 2025 -
Akura Medical raises $53M to advance thrombectomy portfolio
Part of the money will go toward setting up a joint venture in Qatar, whose sovereign wealth fund led the round.
By Susan Kelly • Dec. 3, 2025 -
Medtronic names Jeb Denny as chief medical officer for acute care and monitoring
Denny leads the unit after Medtronic combined its patient monitoring and respiratory businesses last year.
By Elise Reuter • Dec. 3, 2025 -
PitchBook: AI tuck-in deals to drive M&A acceleration in 2026
Buyers will focus on “tuck-ins that add AI or data-driven capabilities or can meaningfully improve scale against emerging competitors,” the analysts said.
By Nick Paul Taylor • Dec. 3, 2025 -
FDA flags risk from dropped BD Alaris pumps after 2 injury reports
BD told customers to immediately remove from use any devices they drop or severely jar.
By Nick Paul Taylor • Dec. 2, 2025 -
CMS finalizes competitive bidding changes to diabetes devices
AdvaMed supported adjustments made from a draft version but still has concerns about the impact on patient access.
By Elise Reuter • Dec. 1, 2025 -
J&J’s US orthopedic leader leaves amid DePuy spinout process
Leslie Storms started a new role on Monday as president of Ethos Veterinary Health.
By Nick Paul Taylor • Dec. 1, 2025 -

 Retrieved from Craig Chandler / University Communication and Marketing / University of Nebraska-Lincoln on November 26, 2025
Retrieved from Craig Chandler / University Communication and Marketing / University of Nebraska-Lincoln on November 26, 2025 Profile
ProfileBuilding a better robot: How Virtual Incision plans to challenge Intuitive
Led by former Intuitive executive Jim Alecxih, Virtual Incision expects the miniaturized design of its robot to stand out in an increasingly crowded field of challengers.
By Susan Kelly • Dec. 1, 2025 -
OIG report finds Medicare overpaying for CGMs and supplies
The CMS could use its competitive bidding program to address price concerns, after payments for the devices swelled over five years, the government watchdog said.
By Elise Reuter • Nov. 26, 2025