Medical Devices: Page 45
-
Abiomed’s latest Impella recall linked to 129 injury, 49 death reports
The J&J subsidiary warned about the risk of the catheter perforating the heart in updated instructions for use. Impella devices were part of four Class I recalls in 2023.
By Elise Reuter • March 21, 2024 -
Insulet names Ana Maria Chadwick CFO
Chadwick succeeds Wayde McMillan, who left last year to become CFO for 3M’s health spinoff.
By Nick Paul Taylor • March 21, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Intuitive aims to keep challengers at bay with new surgical robot
The soft tissue robotics leader looks to set itself apart from upcoming rivals with the first new da Vinci system in 10 years.
By Susan Kelly • March 20, 2024 -
Deka’s automated insulin delivery system, powered by patient-led app, gets FDA clearance
Sequel Med Tech will sell the new system, which integrates with Tidepool’s Loop insulin dosing algorithm.
By Nick Paul Taylor • March 20, 2024 -
FDA stops two manufacturers from importing plastic syringes
The agency issued import alerts to Jiangsu Caina Medical and Jiangsu Shenli Medical Production because their products didn’t meet quality requirements or were different from FDA-authorized versions.
By Nick Paul Taylor • Updated April 8, 2024 -

 Retrieved from Titan on September 12, 2022
Retrieved from Titan on September 12, 2022
Titan Medical to merge with imaging firm Conavi, ending strategic review
The combined company will focus on selling Conavi’s cardiovascular imaging system used in minimally invasive procedures.
By Elise Reuter • March 19, 2024 -
J&J Medtech, Nvidia partner to bring AI to the operating room
The companies see the collaboration as a way to help facilitate the fast, secure deployment of AI algorithms for surgical decision-making.
By Nick Paul Taylor • March 18, 2024 -
Deep Dive
6 ways the FDA can improve medical device recalls
Experts said improving how adverse events are tracked and requiring manufacturers to use electronic notifications could make devices safer.
By Elise Reuter • March 18, 2024 -
Intuitive details launch plan for new da Vinci 5 robot
The fifth generation of the company’s flagship robotic surgery system will be introduced in a phased launch.
By Susan Kelly • March 18, 2024 -
Intuitive lands FDA clearance for new da Vinci robot
The soft tissue robotics leader incorporated new features into the system, from tissue sensing feedback to a smaller size and more comfortable console.
By Susan Kelly • March 15, 2024 -
Fresenius Kabi receives FDA warning letter over issues at ex-Ivenix site
Inspectors found fault with the handling of corrective and preventive actions for the Ivenix Infusion System.
By Nick Paul Taylor • March 15, 2024 -
Q&A
Medtronic’s Sean Salmon on cardiovascular competition, carving out new markets
The president of Medtronic’s cardiovascular portfolio talked about renal denervation, a new procedure to reduce blood pressure and rising competition in cardiac ablation.
By Elise Reuter • March 15, 2024 -
Better Therapeutics lays off staff, considers winding down business
The digital therapeutics developer received authorization for a Type 2 diabetes treatment app last year but has struggled for money.
By Nick Paul Taylor • March 15, 2024 -
EPA final rule limits EtO emissions for medical device sterilizers
Medtech companies now have two years to come into compliance with the new regulations.
By Elise Reuter • March 14, 2024 -
US hospitals predict uptick in procedures as staffing pressures ease
The forecast and a small overall rise in budgets could benefit Boston Scientific, Medtronic and Stryker, BTIG analysts said.
By Nick Paul Taylor • March 14, 2024 -

 Retrieved from Exactech on March 14, 2024
Retrieved from Exactech on March 14, 2024
Exactech hit with warning letter over implant packaging
The FDA found faults in the orthopedics company’s analysis of complaints demonstrating defective packaging could have accelerated wear to its implants.
By Elise Reuter • March 14, 2024 -
Mass General Brigham works with FDA to create brain-computer interface group
The collaborators aim to resolve the clinical, regulatory, coverage and payment questions facing developers of the devices.
By Nick Paul Taylor • March 13, 2024 -
AI to expand medtech portfolios, revenue streams: Moody’s
The rating agency predicts AI will start to have a positive impact on medical device companies in the next two years.
By Nick Paul Taylor • March 12, 2024 -
3M’s board approves health spinoff
Investors will receive one share of Solventum for every four shares of 3M they hold on March 18.
By Nick Paul Taylor • March 12, 2024 -
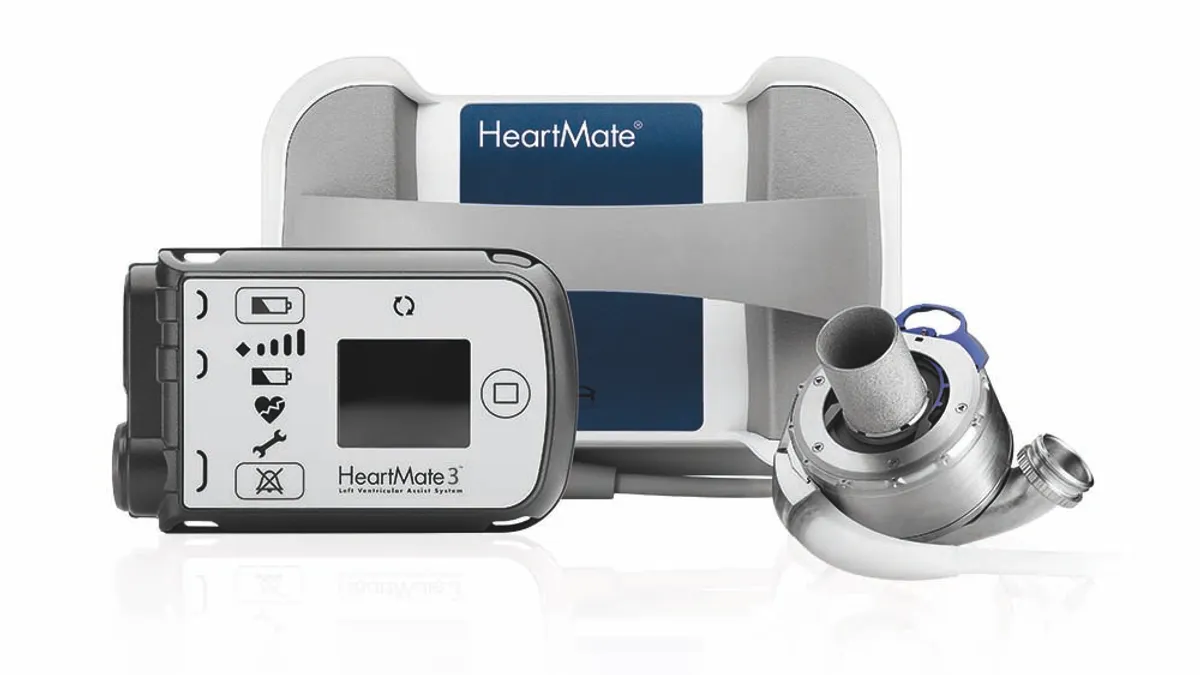
Abbott recalls Heartmate LVAD communication system
The FDA said eight reported injuries have been linked to the problem, which can cause the mechanical heart pump to unexpectedly stop or start.
By Susan Kelly • March 12, 2024 -
3M names William Brown CEO
Michael Roman, who has led 3M since 2018, will step down on May 1.
By Ricky Zipp • March 12, 2024 -
FDA panel backs Lumicell’s agent for breast cancer imaging tool
One panelist who voted yes said the “incremental benefits outweigh the small risks of anaphylaxis.”
By Nick Paul Taylor • March 11, 2024 -
Advamed report finds racial disparities in care, proposes fixes
The report found White patients were more likely to receive several cardiovascular procedures than Black patients, which limits access to medical technology.
By Nick Paul Taylor • March 11, 2024 -
Medtronic recalls more than 45,000 catheter tubing units after injury reports
The issue, which is linked to 26 injuries, could result in neurological harm or death, FDA said.
By Nick Paul Taylor • March 8, 2024 -
Former Stimwave CEO found guilty of selling fake implantable device component
The former executive was convicted on two counts for her role in creating and marketing the faulty devices.
By Susan Kelly • March 8, 2024