Medical Devices: Page 44
-
Smiths Medical recalls thousands of ventilators over fault linked to 8 serious injuries
A problem with the devices can cause patients to receive the wrong amount of ventilation or too little oxygen, the FDA said in a recall notice.
By Nick Paul Taylor • April 5, 2024 -
J&J to acquire Shockwave Medical for $13.1B
Johnson & Johnson has spent more than $30 billion since late 2022 to bolster its cardiac portfolio, buying up Abiomed, Shockwave Medical and Laminar.
By Ricky Zipp • Updated April 5, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
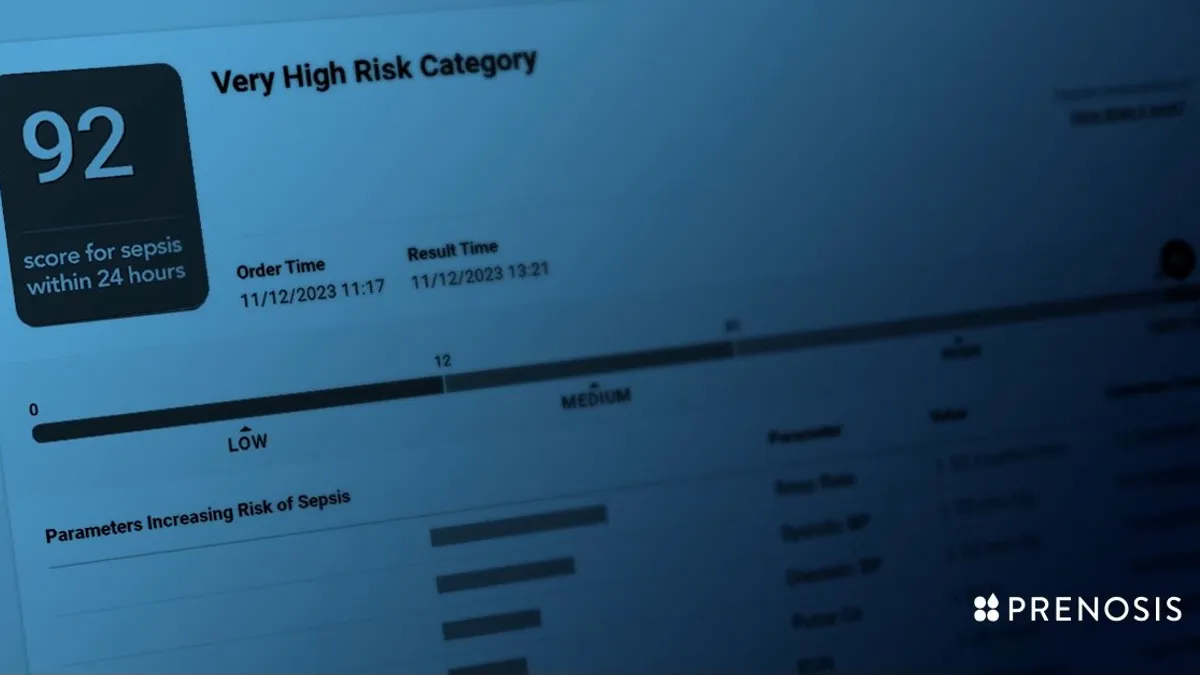
FDA grants de novo nod to AI tool for detecting sepsis
Prenosis CEO Bobby Reddy Jr. told MedTech Dive the company sees third-party validation as important, with the FDA having clarified that certain decision support tools should be regulated as medical devices.
By Elise Reuter • April 4, 2024 -
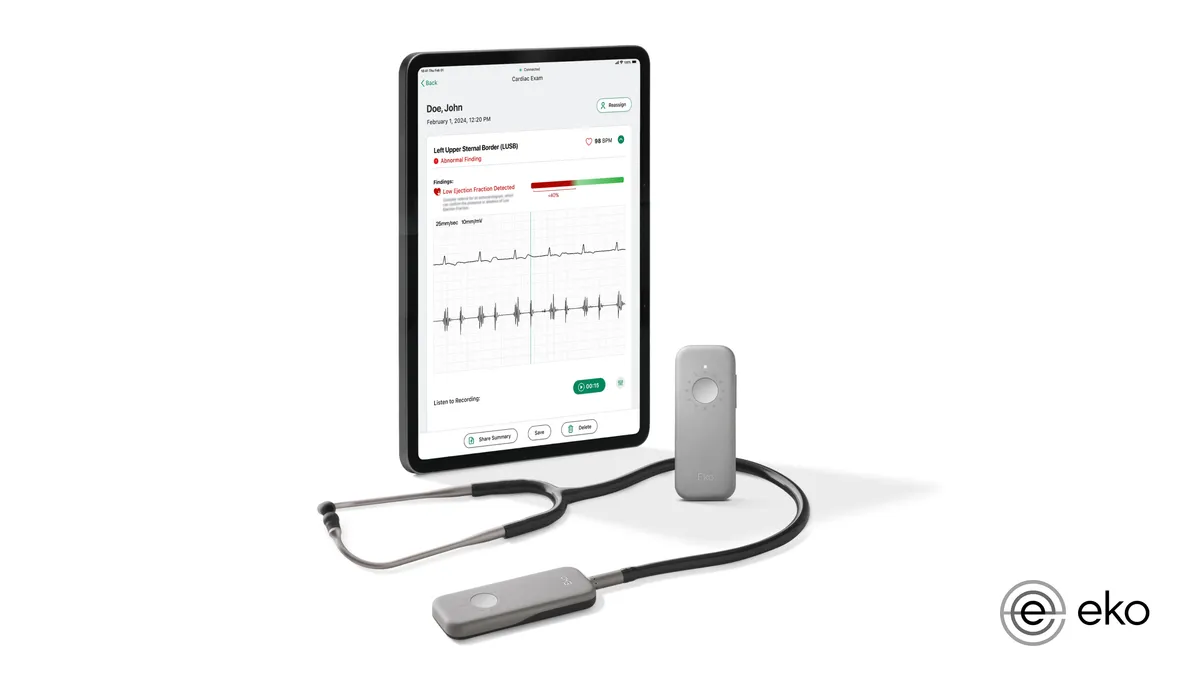
Eko wins FDA nod for AI to detect sign of heart failure using stethoscope
The clearance adds to the list of devices the FDA has authorized this year with AI algorithms to detect health conditions.
By Nick Paul Taylor • April 4, 2024 -
Teleflex catheterization kit recall linked to 10 injuries, 1 death
Nearly 335,000 devices were recalled due to safety issues, which may cause damage to blood vessel walls, artery blockage or death.
By Nick Paul Taylor • April 4, 2024 -
Deep Dive
As cyberattacks on healthcare persist, can the FDA’s new device regs hold up?
Revamped regulations to thwart hackers are a big step forward, but issues such as legacy devices and reliance on software patches pose lingering challenges.
By Ricky Zipp • April 3, 2024 -
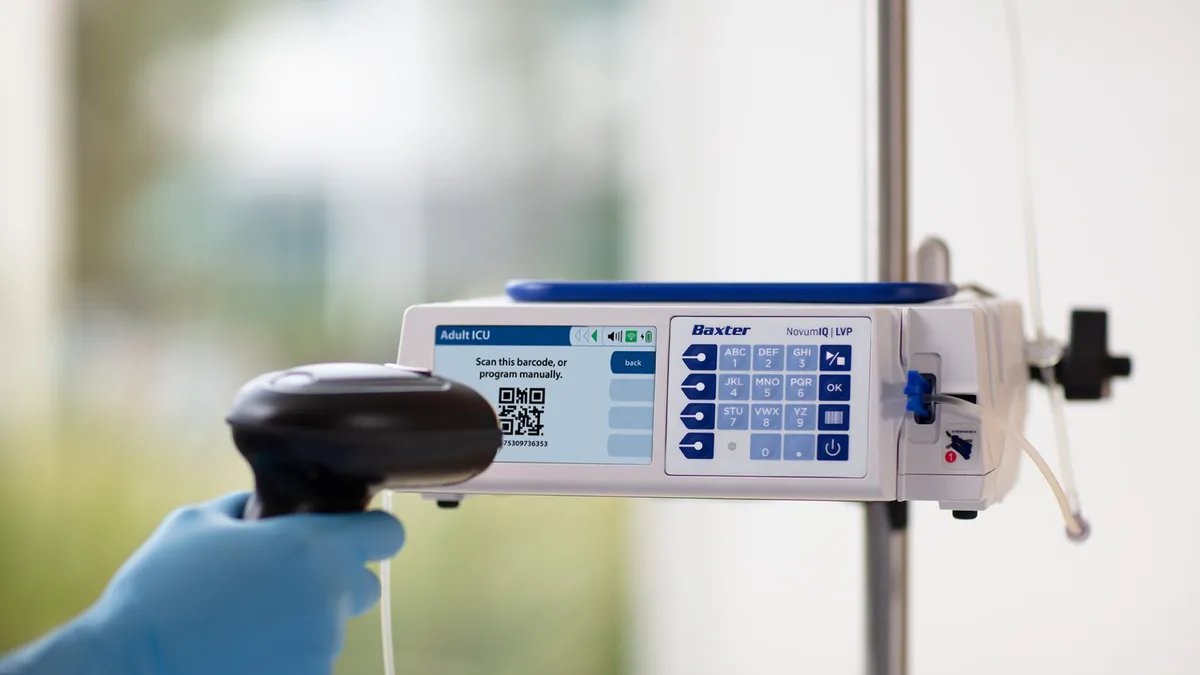
Baxter receives FDA clearance for delayed Novum IQ infusion pump
The clearance ends a three-year back-and-forth with the FDA to get the product to market.
By Nick Paul Taylor • April 2, 2024 -
Abbott nets FDA approval for Triclip
The transcatheter tricuspid valve repair device will compete against Edwards Lifesciences’ Evoque system.
By Susan Kelly • April 2, 2024 -
Abbott wins FDA clearance for bedside blood concussion test
The clearance is a step towards using the test in non-healthcare settings, such as sporting events, Abbott said.
By Nick Paul Taylor • April 2, 2024 -
Infutronix pulls infusion pumps from US after nearly 3,700 complaints
The company listed six issues that can cause the pumps to stop infusions and otherwise malfunction.
By Nick Paul Taylor • April 2, 2024 -
3M spins out Solventum
The new company will need to work through debt from the spinoff and sole-source arrangements with 3M.
By Elise Reuter • April 1, 2024 -
Masimo faces another proxy challenge from Politan Capital
The activist shareholder seeks two more seats on Masimo’s board, after both candidates it nominated last spring were voted in.
By Susan Kelly • April 1, 2024 -
Osso VR to lay off 67 people at corporate HQ
Art and illustration jobs were heavily affected by the layoffs, which will be complete by May 27.
By Nick Paul Taylor • April 1, 2024 -
Q&A
J&J’s Aldo Denti on digital technology in orthopedics
The chairman of J&J subsidiary DePuy Synthes talked about how surgical robots and other technologies have contributed to procedures moving to outpatient facilities.
By Elise Reuter • Updated April 1, 2024 -
Roche wins FDA approval for first molecular malaria blood donor screening test
The company is pitching the test as a way to improve the safety and availability of blood.
By Nick Paul Taylor • March 28, 2024 -
J&J in talks to buy Shockwave Medical: WSJ
The healthcare giant could follow up its $16.6 billion acquisition of Abiomed with another deal for a heart device company, the WSJ reported Tuesday.
By Ricky Zipp • March 27, 2024 -
FDA hits Renovo with warning letter over reprocessed medical devices
The letter accuses Renovo of adding models other than the ones covered by its 510(k) clearances.
By Nick Paul Taylor • March 27, 2024 -
Neuronetics wins FDA clearance for device to treat adolescents with depression
William Blair analysts called the clearance a positive surprise, explaining that the FDA has denied several therapies in the patient population.
By Nick Paul Taylor • March 27, 2024 -
FDA re-issues ban on electric shock devices
The agency tried to ban the devices in 2020, but a federal appeals court overturned the decision.
By Elise Reuter • March 26, 2024 -
Abbott receives CE mark for 6-year insertable cardiac monitor
The device can continuously monitor a patient’s heart rhythms for either three or six years, depending on users’ needs.
By Nick Paul Taylor • March 26, 2024 -
J&J files for FDA approval of Varipulse pulsed field ablation platform
The company wants to catch up with rival PFA systems from Medtronic and Boston Scientific that have already received FDA authorization.
By Nick Paul Taylor • March 26, 2024 -
Masimo to split off consumer business
The decision comes two years after the pulse oximetry specialist acquired Sound United for its audio brands in a $1 billion deal.
By Susan Kelly • March 25, 2024 -
Vyaire Medical recalls Airlife resuscitators over defect linked to 2 death reports
The recall covers respiratory support devices made in 2017 or earlier that can fail to provide enough ventilation.
By Nick Paul Taylor • March 22, 2024 -
Q&A
Stryker’s next head of joint replacement talks about new revision, robotics products
Katherine Truppi will become president of Stryker’s joint replacement business on March 31 after more than 20 years at the company.
By Elise Reuter • March 22, 2024 -
Medtronic claims win in Axonics patent row, pushes for jury trial
New rulings from a U.S. patent appeal board are the latest step in the companies’ intellectual property dispute over sacral neuromodulation devices.
By Nick Paul Taylor • March 22, 2024