Medical Devices: Page 52
-
Hologic promotes Essex Mitchell to COO
Mitchell joined Hologic as vice president of sales and commercial excellence for the GYN surgical division in 2017.
By Nick Paul Taylor • Dec. 11, 2023 -
Medtronic expands AI endoscopy partnership with Cosmo
The collaboration will focus on Cosmo Intelligent Medical Devices’ GI Genius module, which Medtronic distributes globally.
By Elise Reuter • Dec. 11, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Advamed adds medical imaging division, names new board chair
The medtech association is bringing on staff from the Medical Imaging & Technology Alliance to lead the new division.
By Elise Reuter • Dec. 11, 2023 -
BD receives 510(k) clearance for fingerstick blood test sample collection device
The clearance positions BD and partner Babson Diagnostics to support blood collection from community sites such as pharmacies.
By Nick Paul Taylor • Dec. 8, 2023 -
Medtronic secures CE mark for rechargeable Percept deep brain stimulation device
The company plans to make the device available in Western Europe this month and “launch in additional regions based on local regulations.”
By Nick Paul Taylor • Updated Dec. 13, 2023 -
Medtronic scraps plans to buy insulin patch-pump maker EOFlow
Medtronic cited “multiple breaches” of the companies’ agreements. EOFlow has been embroiled in patent lawsuits with Insulet this year.
By Elise Reuter • Dec. 7, 2023 -
5 takeaways from J&J’s investor day
The company outlined its long-term growth expectations and confirmed that it has no plans to separate its medtech and pharma segments.
By Elise Reuter • Dec. 6, 2023 -
US hospitals forecast 9% capex growth to support elective procedure backlog: survey
The Baird survey of 38 health system CFOs forecasts that hospital capital spending growth in 2024 will slow from a 13% jump this year.
By Nick Paul Taylor • Dec. 6, 2023 -
J&J hernia mesh settlement prompts judge to dismiss more than 200 cases
The company and claimants in Georgia filed to dismiss cases related to Ethicon’s Physiomesh in late November.
By Nick Paul Taylor • Dec. 6, 2023 -
Intuitive’s venture capital arm launches second investment fund
The $150 million fund will back early-stage companies focused on healthcare access, digital health, and precision diagnostics and interventions.
By Susan Kelly • Dec. 5, 2023 -
Laboratory trade group, providers oppose FDA’s lab developed test proposal
A CDRH spokesperson confirmed to MedTech Dive that a discrepancy in the submission system used for the proposed rule led to an initial overcount of public comments.
By Nick Paul Taylor • Updated Dec. 7, 2023 -
Baxter sends another safety notice about syringe infusion pump errors
The company shared “reinforced guidance” to mitigate the risk of underdosing and interruptions to treatment.
By Nick Paul Taylor • Updated Dec. 6, 2023 -
Nevro buys low back pain company Vyrsa for $40M
The company expects the acquisition to be revenue accretive starting in 2024.
By Elise Reuter • Dec. 4, 2023 -
Resmed’s new C-suite focused on growing sleep apnea device demand: analysts
After gaining market share during the Philips recall, Resmed can keep growing by stimulating demand through marketing initiatives and new products, William Blair analysts wrote.
By Nick Paul Taylor • Dec. 4, 2023 -
BD tells customers not to use Cardinal’s Monoject syringes with Alaris pumps
The FDA labeled BD's safety notice as a Class I event. The warning comes amid several actions from the agency on the safety of syringes.
By Nick Paul Taylor • Dec. 4, 2023 -
Empatica aims to develop seizure-forecasting algorithm based on wearable data
The company plans a study using its wrist-worn device to capture real-world data that could help understand and predict seizures for people with epilepsy.
By Nick Paul Taylor • Dec. 1, 2023 -
GE HealthCare, Hologic put AI at center of radiology pitches
BTIG analysts said GE HealthCare believes AI can help clinicians and radiologists do their jobs better, which is important due to the “ongoing shortage of radiologists and the high rates of burn out.”
By Nick Paul Taylor • Dec. 1, 2023 -
Q&A
Moon Surgical CEO Anne Osdoit on readying a soft tissue robot for launch
With support from J&J’s venture capital arm and chip maker Nvidia, Moon is targeting a soft tissue surgery market dominated by Intuitive Surgical.
By Susan Kelly • Dec. 1, 2023 -
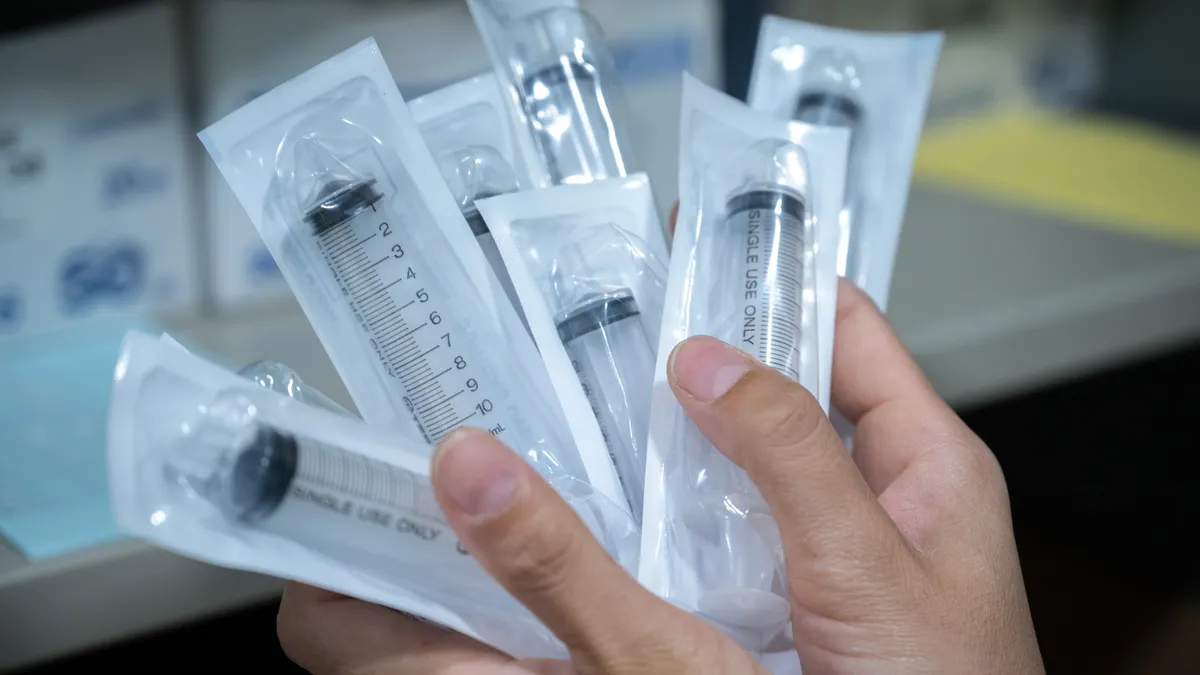
 Retrieved from Food and Drug Administration on December 01, 2023
Retrieved from Food and Drug Administration on December 01, 2023
FDA advises against using plastic syringes made in China amid reports of quality failures
Officials began the investigation after receiving information about quality issues associated with “several Chinese manufacturers of syringes.”
By Nick Paul Taylor • Dec. 1, 2023 -
J&J heats up LAA closure competition with $400M Laminar buy
Just days after Medtronic announced it would launch a LAA device, J&J said it has acquired a company that is developing a new treatment for LAA closure.
By Susan Kelly • Nov. 30, 2023 -
Vivos receives FDA 510(k) clearance for oral devices to treat severe sleep apnea
The clearance positions the company to offer an alternative to continuous positive airway pressure devices and neurostimulation implants.
By Nick Paul Taylor • Nov. 30, 2023 -
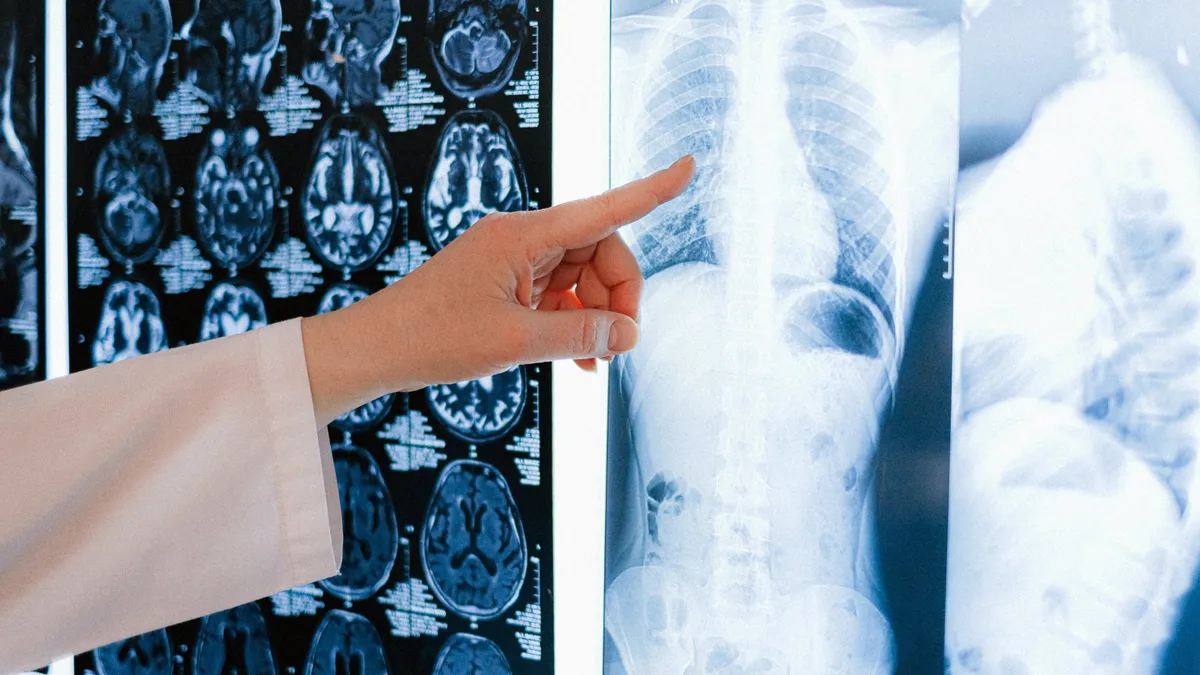

Anna Shvets via Pexels

GE HealthCare gets 510(k) clearance for algorithm to detect collapsed lungs
The system provides immediate notifications to support the triaging of emergency room patients.
By Nick Paul Taylor • Nov. 29, 2023 -
3 medtech spinoffs that reshaped the industry in 2023, and what to expect next
GE HealthCare, Johnson & Johnson and Danaher completed transactions this year. Baxter, 3M and Medtronic are now on deck.
By Susan Kelly • Nov. 29, 2023 -
Advamed applauds Biden’s new actions to strengthen U.S. supply chains
The actions include using the Defense Production Act to enable investment in U.S. manufacturing of essential medicines and medical countermeasures, such as diagnostics.
By Nick Paul Taylor • Nov. 29, 2023 -
‘Fire, smoke, burns’: FDA warns about new safety risks for Philips CPAP machines
Philips recently filed more than 270 reports of thermal problems related to its DreamStation 2 device from the past three years.
By Elise Reuter • Nov. 28, 2023