Medical Devices: Page 55
-
Stryker in ‘sprint back to 2019’ as procedures grow and supply chain, staffing pressures improve
The company expects a procedure backlog to boost orthopedic surgery demand in the fourth quarter and through 2024.
By Ricky Zipp • Nov. 3, 2023 -
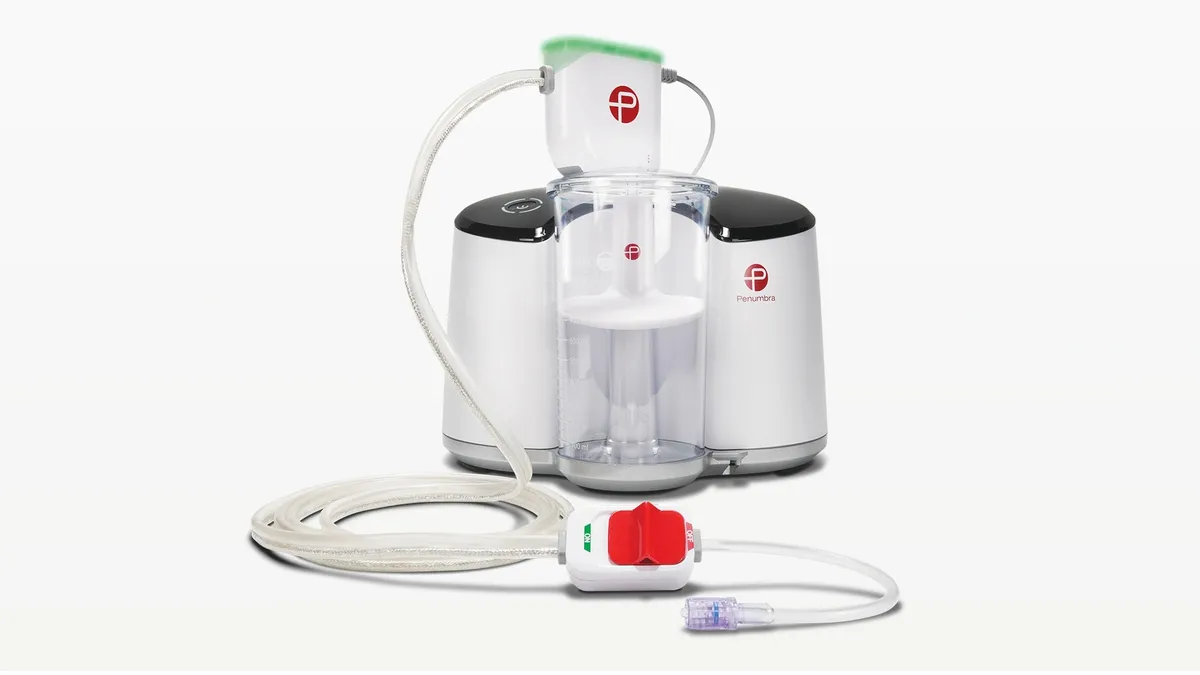
Penumbra touts benefits of vacuum thrombectomy for PE in late-breaking data
An interim analysis of Penumbra’s Indigo aspiration system met its primary safety and efficacy criteria, according to data shared at the Vascular Interventional Advances Conference.
By Elise Reuter • Nov. 3, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
 Retrieved from Google on May 28, 2020
Retrieved from Google on May 28, 2020
Elective procedure interest still topping pre-COVID levels, survey finds
Google searches for 20 elective surgeries in the U.S. were at 112% of pre-COVID levels in the week ended Oct. 28, according to a report from Needham analysts.
By Susan Kelly • Nov. 3, 2023 -
Inari Medical to buy limb-saving device maker LimFlow for up to $415M
The deal includes $250 million in cash upfront and additional payments based on reaching commercial and reimbursement milestones.
By Susan Kelly • Nov. 3, 2023 -
Baxter completes biopharma solutions sale, business unit revamp
The company plans to use most of the $3.7 billion in after-tax proceeds from the biopharma solutions sale to repay debt.
By Susan Kelly • Nov. 2, 2023 -
CooperCompanies buys reproductive health products from Cook Medical after failed takeover
The company paid $300 million to buy certain maternal fetal medicine and gynecological surgery products.
By Elise Reuter • Nov. 2, 2023 -
FDA extends pandemic-era policy allowing certain device, production changes without prior approval
Companies can make limited modifications on certain devices and manufacturing processes to address supply shortages or production limitations.
By Ricky Zipp • Nov. 2, 2023 -
Drilling down on obesity drugs: What medtech executives are saying
Investor concerns that interest in GLP-1s will slow demand for medical devices and procedures have shaved roughly $370 billion in value from stocks across the medtech sector, according to research by Mizuho.
By Susan Kelly • Nov. 2, 2023 -
White House orders HHS to start collecting reports on the safety of AI in healthcare
President Joe Biden outlined the planned program as part of an executive order on “safe, secure, and trustworthy” artificial intelligence.
By Nick Paul Taylor • Nov. 1, 2023 -
Q&A
Stryker’s Lisa Kloes talks cementless implants, robotics as future of knee business
The manager of Stryker’s knee business also discussed the growing role of ambulatory surgery centers in orthopedics.
By Elise Reuter • Updated Nov. 8, 2023 -
FDA warning letter accuses Wavi of selling unapproved neurological device
Wavi markets its headset as providing a baseline scan for patients who have a traumatic event, an injury or are worried about their brain as they age.
By Nick Paul Taylor • Nov. 1, 2023 -
GE HealthCare sales resilient despite ‘highly volatile’ economy
The company raised its earnings forecast for 2023 as it outlined plans to expand margins and navigate China's anti-corruption campaign.
By Elise Reuter • Oct. 31, 2023 -
11 key moments in Philips’ massive recall of respiratory devices
The recall of millions of sleep apnea devices and ventilators is still ongoing more than two years later, as Philips works to repair and replace affected machines.
By Elise Reuter • Oct. 31, 2023 -
Tracker
Tracking Philips Respironics recalls
Philips recalled its Trilogy ventilators to fix new and previously reported problems with the machines. The company issued a mandatory software update and changed the devices’ instructions.
By Elise Reuter • Updated Oct. 3, 2024 -
FDA posts updated safety data on Bayer’s Essure, notes progress on improving study
The agency removed the “inadequate” progress tag it applied last year after Bayer acted to get people back in the study.
By Nick Paul Taylor • Oct. 31, 2023 -
4 medtech companies to watch this week in earnings
GE HealthCare and Stryker are among the top medtech companies posting financial results in the third week of this earnings season.
By Susan Kelly • Oct. 30, 2023 -
Resmed cuts 5% of staff amid profitability push
The company has stopped some projects that were not working well as it seeks to cut operating costs, CEO Mick Farrell told investors.
By Elise Reuter • Oct. 30, 2023 -
Philips leader signed off on sale of respiratory devices despite evidence of health risks: report
Roy Jakobs approved the sale of respiratory devices held by manufacturers after the company stopped shipping new machines, according to an article published last week by Pittsburgh Post-Gazette and ProPublica.
By Nick Paul Taylor • Oct. 30, 2023 -
Masimo prevails against Apple in USITC patent investigation
The patent dispute over pulse oximetry functions could result in a ban on imports of some Apple Watches.
By Susan Kelly • Oct. 27, 2023 -
Edwards posts early look at tricuspid device, stoking Abbott rivalry ahead of FDA panel
Nearly 94% of patients had their condition improve to mild or no tricuspid regurgitation six months after treatment, according to the study.
By Nick Paul Taylor • Oct. 27, 2023 -
Q&A
Baxter’s Heather Knight talks combating sepsis — and how AI can help
The company’s group president for medical products and therapies says the right fluid management practices can help save lives and lower hospital costs.
By Susan Kelly • Oct. 27, 2023 -
GE HealthCare’s imaging AI will become ‘increasingly meaningful’ to growth: Moody’s
Even if modestly accretive, AI could support longer-term revenue targets at GE HealthCare and its competitors, Moody’s analysts wrote.
By Nick Paul Taylor • Oct. 27, 2023 -
Accuray lays off nearly 6% of radiotherapy workforce
The company delivered fourth quarter results that fell short of analyst expectations in August, causing its share price to fall.
By Nick Paul Taylor • Oct. 27, 2023 -
Boston Scientific looks to new devices in 2024 to drive growth
The company expects to receive FDA approval for its Agent drug-coated balloon and its Farapulse pulsed-field ablation system next year.
By Elise Reuter • Oct. 26, 2023 -
Abbott’s resorbable scaffold improves outcomes in below-the-knee artery disease, study finds
The device maker plans to submit the Esprit BTK data to the Food and Drug Administration for review.
By Nick Paul Taylor • Oct. 26, 2023