Medical Devices: Page 58
-
Baxter hires new CFO from food industry
Joel Grade will join the company after 25 years at Sysco.
By Elise Reuter • Oct. 3, 2023 -
FDA expands total product life cycle program to cover neurological devices
The agency could enroll up to 45 additional devices over the coming year.
By Nick Paul Taylor • Oct. 3, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA details switch to electronic filings for 510(k), de novo submissions
The guidance documents advance the transition to eSTAR, an electronic template the FDA is adopting to meet legislative requirements.
By Nick Paul Taylor • Oct. 3, 2023 -
Opinion
Friend, not foe: How AI will revolutionize patient care
Artificial intelligence should be embraced to help streamline data and tackle clinician burnout, says Medtronic’s Stacey Churchwell.
By Stacey Churchwell • Oct. 2, 2023 -
Accuray’s Suzanne Winter to lead AdvaMed radiotherapy sector
Winter, the CEO of Accuray, succeeds Baxter’s Chris Toth as chair of the trade group’s radiation therapy sector.
By Susan Kelly • Oct. 2, 2023 -
Medtronic in exclusive talks for $7B sale of units to private equity: report
The Carlyle Group is leading the race to buy Medtronic’s connected patient monitoring and respiratory care operations, Reuters reported.
By Nick Paul Taylor • Oct. 2, 2023 -
Sponsored by Esper
Healthtech companies dealing with complex device fleets, new report finds
Four ways HealthTech companies are using modern solutions to manage their mission-critical devices.
Oct. 2, 2023 -
FDA to regulate lab-developed tests under proposed rule
The tests have come under scrutiny by the agency and lawmakers, but the FDA is still likely to face pushback on the changes.
By Elise Reuter • Sept. 29, 2023 -
Abbott data suggests GLP-1 drugs a ‘modest accelerator’ for CGMs: analysts
The analysis of U.S. insurance claims found people on GLP-1 therapy wear their FreeStyle Libre sensors more.
By Nick Paul Taylor • Sept. 29, 2023 -
As government shutdown looms, medical device reviews expected to continue
The FDA could furlough nearly a fifth of its staff by Sunday if Congress doesn’t reach a funding agreement.
By Elise Reuter • Sept. 28, 2023 -

 Retrieved from Galen Robotics on September 27, 2023
Retrieved from Galen Robotics on September 27, 2023
Galen robot gets FDA de novo clearance for ENT surgeries
The startup affiliated with Johns Hopkins University said it is opening a bridge round for its series B financing as it targets more applications for the robotic surgery assistant.
By Susan Kelly • Sept. 28, 2023 -
Artificial heart maker’s cash runway shrinks to 1 month after supply woes hurt sales
France’s Carmat expects revenues of up to 6 million euros in the second half of the year but needs to secure financing to avoid running out of money.
By Nick Paul Taylor • Sept. 28, 2023 -
Harbinger Health raises $140M to study blood-based cancer screening test
The test developer will use the series B funding to complete a 10,000-subject clinical trial ahead of a planned launch in 2025.
By Nick Paul Taylor • Sept. 28, 2023 -
Siemens Healthineers buys Aspekt to bolster radiation oncology services
The company’s Varian unit sees Aspekt as complementary to its Advanced Oncology Solutions business, which provides professional and clinical services.
By Nick Paul Taylor • Sept. 27, 2023 -
FDA finalizes guidance on cybersecurity for medical devices
Congress granted the agency authority to deny premarket submissions that lack cybersecurity information, starting in October.
By Nick Paul Taylor • Sept. 27, 2023 -
Device component-maker Zeus exploring sale: Reuters
The firm, which manufacturers catheters and other medical components, could be valued at $4 billion or more, according to the report.
By Elise Reuter • Sept. 26, 2023 -
Edwards Lifesciences says it was target of surprise EU antitrust inspection
The European Commission announced the action last week without naming the cardiovascular device company.
By Susan Kelly • Sept. 26, 2023 -
Surgical robot maker Vicarious faces possible NYSE delisting
The company’s shares could be delisted from the New York Stock Exchange if the price does not rise above $1.
By Elise Reuter • Sept. 26, 2023 -
Clinical data from Boston Scientific and rivals could reshape pulmonary embolism market: analysts
It is not yet clear how different approaches would benefit specific patients, creating uncertainty about which businesses will profit from the shift.
By Nick Paul Taylor • Sept. 26, 2023 -
Enovis inks $847M acquisition to boost orthopedic reconstruction business
A Wells Fargo analyst said the deal was “much larger than what we were expecting.”
By Nick Paul Taylor • Sept. 26, 2023 -
Intarcia’s diabetes drug-device combo voted down again by FDA panel
The 19-member advisory committee unanimously voted against Intarcia in the review, citing cardiovascular and kidney safety concerns.
By Gwendolyn Wu • Sept. 25, 2023 -
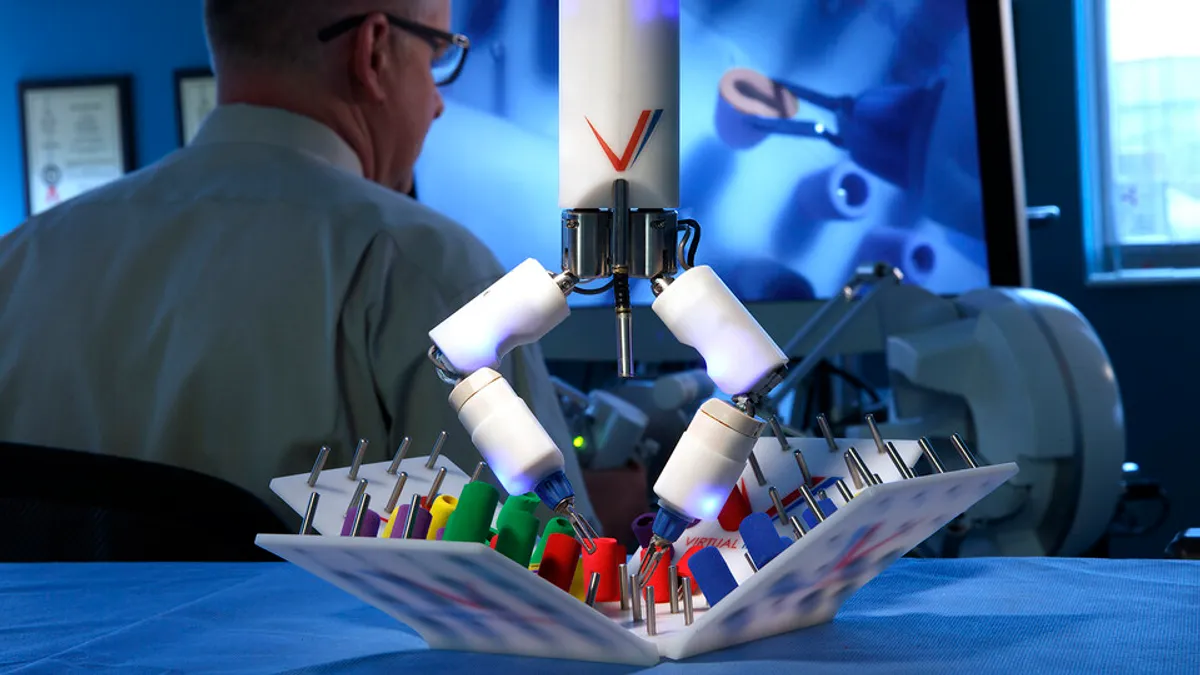
 Retrieved from University of Nebraska-Lincoln on September 25, 2023
Retrieved from University of Nebraska-Lincoln on September 25, 2023
Developer of miniaturized surgical robot extends funding round
Virtual Incision, whose surgical robot is headed to the International Space Station next year, raised $30 million in an extension of its series C round.
By Susan Kelly • Sept. 25, 2023 -
Medtronic receives CE mark for CGM sensor, boosting challenge to Abbott and Dexcom
Medtronic has made the sensor smaller and eliminated the need for fingersticks to calibrate the device, bringing a challenger to market leaders Abbott and Dexcom.
By Nick Paul Taylor • Sept. 25, 2023 -
FDA shares draft medical device harmonization plan to meet MDUFA V goal
The FDA outlined deadlines for actions it will take, including creating a mechanism for working with regulatory partners.
By Nick Paul Taylor • Sept. 25, 2023 -
Sponsored by Shyft Global Services
How can medical device OEMs support the circular economy?
Discover how medical device OEMs can be part of the circular economy for a more sustainable future.
Sept. 25, 2023