Medical Devices: Page 68
-
Medtronic posts profit, sales growth in Q4, agrees to buy Korean diabetes patch maker
The company is “confident in delivering durable revenue growth in the year ahead” amid continued improvements in procedure volumes.
By Peter Green • May 25, 2023 -
Slump in medtech VC activity continues as inflation keeps investors on the sidelines
Investors struck deals worth $1.8 billion over the first three months of the year, down from $3.0 billion across the start of 2022.
By Nick Paul Taylor • May 25, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Acurable prepares to challenge ResMed for sleep apnea diagnosis market
The company’s device records the sounds of the patient’s respiratory and cardiac functions and monitors blood oxygen levels to assess suspected obstructive sleep apnea.
By Nick Paul Taylor • May 25, 2023 -
FDA teams with Veterans Health Administration to make medtech supply chains more resilient
Rather than amassing reserves of physical supplies, “digital stockpiles” would create a network of trusted suppliers that have the ability to make products at or near the point of use.
By Nick Paul Taylor • May 24, 2023 -
Integra recalls all devices made at Boston facility in the past 5 years, pauses production
Integra initiated the recall after an internal investigation found endotoxin testing deviations that can result in postoperative fevers. Analysts at BTIG said the recall raises “bigger questions” about Integra’s management.
By Nick Paul Taylor • May 24, 2023 -
‘Genuine bull market’ underway in medtech stocks, says CNBC’s Cramer
The “Mad Money” show host said shares of medical device companies stand to benefit from pent-up demand for surgeries postponed during the COVID-19 pandemic.
By Susan Kelly • Updated May 23, 2023 -
Orthopedic markets to return to normal this year as post-pandemic boost fades: analysts
Hip growth should normalize late this year, while the knee and spine markets are expected to slow down by the end of 2024, say analysts at Needham.
By Nick Paul Taylor • May 23, 2023 -
PulseAI algorithm beats Apple Watch software at detecting atrial arrhythmias
In a head-to-head comparison with Apple’s pre-installed software for interpreting ECG data, Pulse AI’s algorithm was better at detecting atrial arrhythmias.
By Nick Paul Taylor • May 23, 2023 -
Vicarious, preparing to take on Intuitive in robotic surgery, names new COO
John Mazzola will oversee operations as Vicarious prepares to seek FDA approval for a robotic surgery system that would take on Intuitive Surgical’s market-leading da Vinci robot.
By Susan Kelly • May 22, 2023 -
Beta Bionics wins FDA nod to challenge Medtronic for automated insulin pump market
Beta Bionics’ device, aimed at people with Type 1 diabetes, has eliminated most of the data entry required by competitors.
By Nick Paul Taylor • May 22, 2023 -
Abbott, Boston Scientific, Medtronic share data on heart rhythm devices at conference
A series of recent innovations in pacemaker technology has opened up new markets and set the stage for increased competition.
By Nick Paul Taylor • May 22, 2023 -
A Boston Scientific purchase of Shockwave could be transformative — but at what price?
Boston Scientific has used “tuck-in” acquisitions to boost growth in the past. Some analysts say it should stay the course amid a report the company may make a bid.
By Susan Kelly • May 19, 2023 -
Precision Lens ordered to pay $487M for kickbacks to eye surgeons in Medicare false-claims case
Luxury vacations and tickets to Broadway shows were used by the Minnesota-based ophthalmologic supply company to induce surgeons to use its products, a federal court ruled.
By Elise Reuter • May 19, 2023 -
Abbott gains edge over cardiac monitor rivals with FDA clearance of 6-year device
Rival devices sold by Biotronik, Boston Scientific and Medtronic have battery lives ranging from three to 4.5 years.
By Nick Paul Taylor • May 19, 2023 -
Chinese medtechs post data on renal denervation devices to rival Medtronic, ReCor
The findings validate the technique used by Medtronic and ReCor, while suggesting the U.S. companies will face competition if they target the Chinese market.
By Nick Paul Taylor • May 19, 2023 -
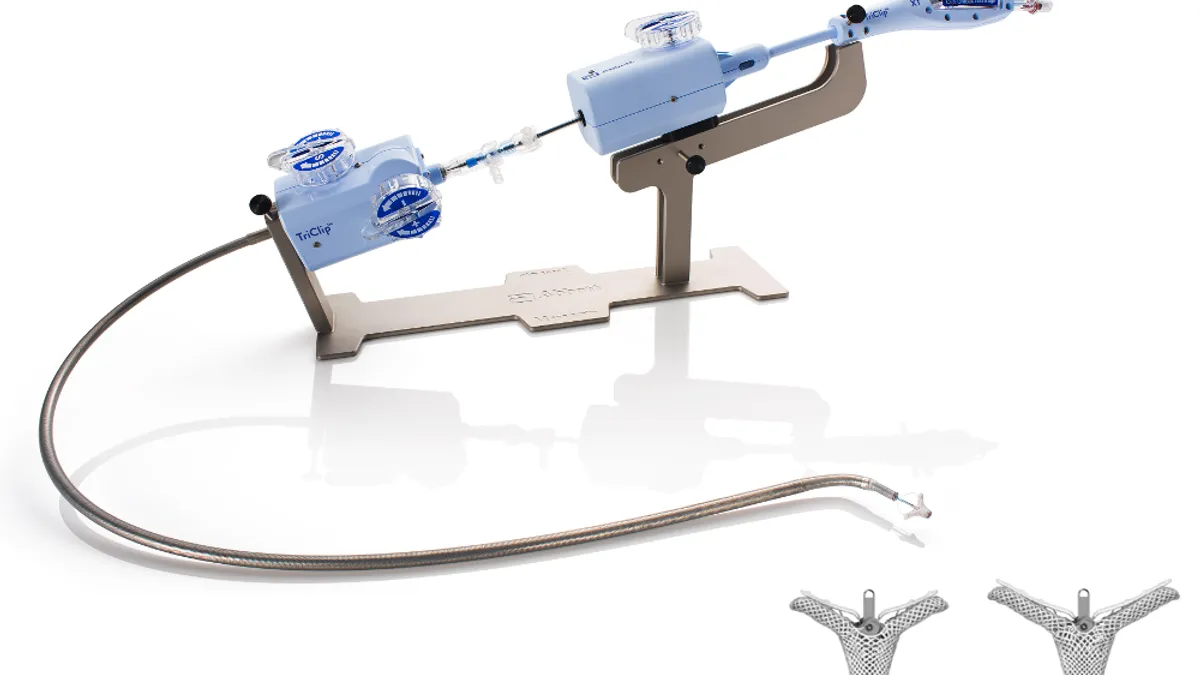
Abbott shares data showing benefit of TriClip device amid FDA review
Patients experienced a reduction in tricuspid regurgitation and had improved heart function, according to late-breaking data presented at EuroPCR.
By Elise Reuter • May 18, 2023 -
J&J names Jasmina Brooks as president of Biosense Webster
Brooks will lead the company as J&J competes with rivals such as Boston Scientific over cardiac ablation technology.
By Nick Paul Taylor • May 18, 2023 -
Clinical trials showcase potential roles for ultrasound in treating cancer, inflammatory disease
The use of ultrasound as a therapeutic device could lead to new treatment options. In one study, patients with brain tumors received an ultrasound implant that allowed a drug to enter the brain.
By Nick Paul Taylor • May 18, 2023 -
Opinion
To lead in medtech, apply best practices from direct-to-consumer businesses
Entrepreneurs who are willing to leverage proven DTC strategies can gain a competitive advantage in medtech, argues Scott Nelson, president and CEO of FastWave Medical.
By Scott Nelson • May 17, 2023 -
Rapid AI adoption could cause medical errors, patient harm, WHO warns, urging oversight
Warning that caution is not being exercised with use of artificial intelligence in healthcare, the World Health Organization called for rigorous oversight of the new technology.
By Susan Kelly • May 17, 2023 -
ITC to review ruling that Apple violated Masimo’s patents
If the ITC finds Apple improperly used Masimo’s pulse oximetry technology in the Apple Watch, the commission could ban imports of some Watch versions.
By Elise Reuter • May 17, 2023 -
Abbott wins FDA spinal cord stimulation approval to challenge Nevro for back pain market
The label expansion comes 16 months after Nevro won FDA approval in non-surgical refractory back pain and four months after Boston Scientific released data on its rival device.
By Nick Paul Taylor • Updated May 17, 2023 -
Philips claims exposure to foam particles in recalled sleep apnea devices unlikely to harm patients
Inhaling foam particles or chemicals from the recalled devices is unlikely to significantly harm patients, Philips argued, but it added that regulators may reach a different conclusion.
By Elise Reuter • Updated May 16, 2023 -
Intuitive Surgical promotes Dave Rosa to president, Guthart remains CEO
The surgical robot maker said longtime CEO Gary Guthart retains his role and will continue to lead the company.
By Susan Kelly • May 16, 2023 -
Cook Medical lays off 500 after ‘significant change’ to operating environment
In a memo to employees, CEO Pete Yonkman said that hourly manufacturing and distribution workers won’t be laid off because demand for Cook Medical’s products “continues to grow.”
By Nick Paul Taylor • May 16, 2023