Medical Devices: Page 71
-
Fresenius flags fault with infusion system acquired last year in $240M deal
Fresenius noted a problem with the devices that can cause loss of power, potentially delaying or interrupting the supply of critical fluids and medications.
By Nick Paul Taylor • April 20, 2023 -
J&J study data show dramatic benefit to multiple myeloma cell therapy
A study abstract inadvertently posted online showed J&J and Legend’s therapy, Carvykti, reduced the risk of disease progression or death by more than 70% over standard drugs.
By Ned Pagliarulo • April 19, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medtronic says it plans more layoffs, declines to specify locations
The company said it began notifying some employees on Tuesday, but would not confirm the number of positions or locations of the jobs that would be cut.
By Elise Reuter • April 19, 2023 -
Abbott boosts organic sales outlook as device demand accelerates
A rebound in surgical procedure volumes as hospital staffing improved at the start of the year partially offset a steep drop in COVID-19 testing sales.
By Susan Kelly • April 19, 2023 -
AFib study shows no effect on outcomes from 1-year delay of catheter ablation
Early treatment could be a boon for medtech companies such as Boston Scientific and Medtronic but is potentially challenging for healthcare systems. The new study suggests waits of up to a year will not harm patients.
By Nick Paul Taylor • April 19, 2023 -
Intuitive posted 26% jump in robotic procedures in Q1 as hospitals chipped away at backlog
The company experienced “some backlog effect from patients generally returning to more normalized healthcare routines, given the effect of the pandemic over several years.”
By Nick Paul Taylor • April 19, 2023 -
J&J raises forecast as medtech procedures recover
The company’s medical device business grew by more than 7% in the first quarter.
By Elise Reuter • April 18, 2023 -
Post-approval modification of high-risk devices linked to 30% jump in recall risk: study
The authors suggest improved post-marketing surveillance systems may be needed to mitigate risks to patient safety.
By Nick Paul Taylor • April 18, 2023 -
What medtech startups need to know about remote monitoring
Four medtech executives talked about the future of hospital at home, and the difference between consumer devices and medical devices, in an event hosted by the Colorado Bioscience Association.
By Elise Reuter • April 17, 2023 -
LivaNova CEO quits as firm announces higher-than-expected preliminary earnings
Analysts from Needham and Stifel had opposite takes on how McDonald’s departure will affect the company, which has struggled in recent years to bring new products to market.
By Peter Green • April 17, 2023 -
Abbott gets clearance for Libre 3 reader, opening path to Medicare coverage
With a standalone reader, Abbott said it is working to make the FreeStyle Libre 3 system available to people covered by Medicare.
By Elise Reuter • April 17, 2023 -
Sponsored by Esper
Overcome the top 5 device management challenges of remote patient care with ease
As more organizations adopt RPM, they’re facing increasingly complex challenges managing, updating and securing their devices.
April 17, 2023 -
Proxy adviser backs diabetes device developer Sernova in fight against activist investors
Institutional Shareholders Services said the activist investors “failed to present a detailed explanation” of their case and recommended that shareholders vote for Sernova’s director nominees.
By Nick Paul Taylor • April 17, 2023 -
Q&A
Friday Q&A: Medtronic’s Ajizian talks connected care ahead of unit spinoff
From supporting AI-driven medical interventions to freeing up providers from administrative tasks, remote patient monitoring is raising the bar for care, says Medtronic’s CMO for patient monitoring.
By Susan Kelly • April 15, 2023 -
Medtronic, 6 months after proposing patient monitoring spinoff, remains mum on separation plan
A standalone entity could achieve faster growth, even though recent spinoffs in the medtech sector have produced mixed results, analysts say.
By Susan Kelly • April 14, 2023 -
Fewer Philips replacement devices have reached patients than counted, FDA says
The agency raised concerns that the discrepancy could affect the estimated wait times for patients needing a replacement sleep apnea device.
By Elise Reuter • April 14, 2023 -
Medtronic targets June closure of Epix facility, will lay off 59 employees
The company had bought Epix for a catheter-based, temperature-controlled cardiac ablation system designed to treat patients with irregular heartbeats.
By Nick Paul Taylor • April 14, 2023 -
Digital surgery firm Surgalign faces delisting by Nasdaq for shareholder equity noncompliance
The exchange has given Surgalign 45 days to submit a plan to regain compliance.
By Nick Paul Taylor • April 14, 2023 -
OTC hearing aid matches performance of audiologist-fitted device in small trial
People who used an over-the-counter hearing aid for six weeks saw similar results to people who used an audiologist-fitted device, suggesting less costly, self-fitted hearing aids could be an option.
By Nick Paul Taylor • April 13, 2023 -
Medtech sector poised for growth as healthcare staffing tops pre-COVID levels: KeyBanc
The analysts are “leaning into increased medtech exposure” after determining that several challenges that have constrained the industry are easing.
By Nick Paul Taylor • April 13, 2023 -
Zimmer’s ‘smart knee’ implant may qualify for new technology payments from CMS
If finalized, the add-on payments would help cover the cost of the sensor technology included in the company’s Persona IQ knee implants.
By Elise Reuter • April 12, 2023 -
FDA starts device sterilization pilot program to help industry adapt to EtO emission clampdown
The FDA “recognizes the need to facilitate more timely changes” amid a push to cut EtO emissions and constraints on the supply of radioactive cobalt.
By Nick Paul Taylor • April 12, 2023 -
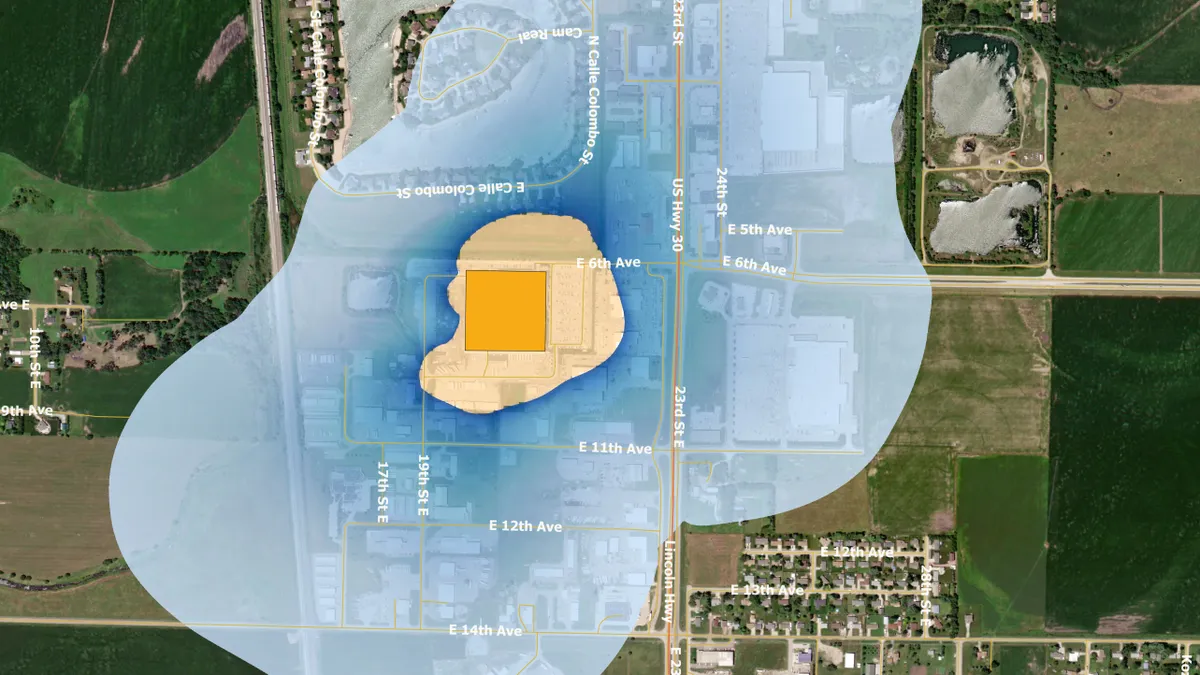
 EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
Medical device groups say ‘the stakes are high’ with new EtO regulations
AdvaMed, which represents medical device companies, warned that patients could face treatment delays if sterilization facilities close.
By Elise Reuter • April 11, 2023 -
EPA to limit ethylene oxide emissions from medical device sterilizers
The agency proposed new emissions limits that device sterilization companies would have 18 months to meet.
By Elise Reuter • April 11, 2023 -
GE HealthCare faces ‘competition from all angles’ that could constrain growth: analysts
BTIG lists 20 companies as competitors to GE HealthCare, including China’s Shenzhen Mindray Bio-Medical Electronics and Shanghai United Imaging Healthcare.
By Nick Paul Taylor • April 11, 2023