Medical Devices: Page 72
-
Medtech buyouts by private equity in Q4 shed light on deal-making trends in 2023: report
Medtech companies are continuing to spin off assets to realign portfolios, creating opportunities for private equity firms to buy businesses and exit existing investments.
By Nick Paul Taylor • April 11, 2023 -
Philips recalls 1,200 reworked sleep apnea devices over fault that can cause therapy failure
The problems keep mounting for Philips, as recently repaired respirators now need to be fixed in a recall the FDA has labeled a Class I event.
By Nick Paul Taylor • April 10, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
EPA proposes stricter ethylene oxide pollutant rules for chemical makers
Further EPA proposals involving medical device sterilization plants are expected to be forthcoming.
By Susan Kelly • April 7, 2023 -
Q&A
Friday Q&A: Fujifilm’s Henry Izawa on navigating a tough hospital equipment market
The CEO of Fujifilm Healthcare Americas discusses integrating Hitachi’s diagnostic imaging business, new product launches and how hospital budgets are changing the market for imaging.
By Elise Reuter • April 7, 2023 -
Sernova defends record after investors attack ‘sluggish’ progress of regenerative medicine implant
The investors accused Sernova’s board of “complacently” overseeing “a period of eroding shareholder value.”
By Nick Paul Taylor • April 7, 2023 -
 Retrieved from Abbott on April 06, 2023
Retrieved from Abbott on April 06, 2023
Abbott faces Class I recall of 4.2M glucose readers on battery fire risks
Readers for Abbott’s FreeStyle Libre glucose monitoring systems could catch fire if improperly stored or charged, the FDA says.
By Peter Green • Updated April 7, 2023 -
Bioventus CEO departs after CartiHeal acquisition unravels
Ken Reali was asked to leave after a deal to buy CartiHeal fell apart. Bioventus’ share price has fallen more than 90% in the past two years.
By Susan Kelly • April 6, 2023 -
Medtech servicing timelines and costs improve as technician turnover creates skills challenge
With experienced technicians retiring faster than their replacements are getting up to speed, the sector faces a potentially costly skills challenge.
By Nick Paul Taylor • April 6, 2023 -
Medtronic’s Hugo too similar to Intuitive’s surgical robot to displace the market incumbent: analysts
The analysts expect Medtronic to “find a place in specific markets” that Intuitive is yet to dominate.
By Nick Paul Taylor • April 5, 2023 -
Philips Trilogy Evo ventilators face another Class I recall
More trouble for Philips’ Respironics business as the company flags a problem with ventilators that could lead to under-delivery of oxygen due to inaccurate readings.
By Elise Reuter • April 4, 2023 -
Masimo receives De Novo nod from FDA for device designed to detect signs of opioid overdose
The system detects patterns associated with opioid-induced respiratory depression and sends alerts that escalate in line with the risk level.
By Nick Paul Taylor • April 4, 2023 -
Bain acquires Olympus life sciences unit for $3.1B
The company aims to expand its position as a workflow solution provider in life science and industrial markets.
By Susan Kelly • April 3, 2023 -
Medtronic, DaVita launch kidney care joint venture
The new company, called Mozarc Medical, will focus on kidney care technology including home dialysis.
By Elise Reuter • April 3, 2023 -
Deep Dive
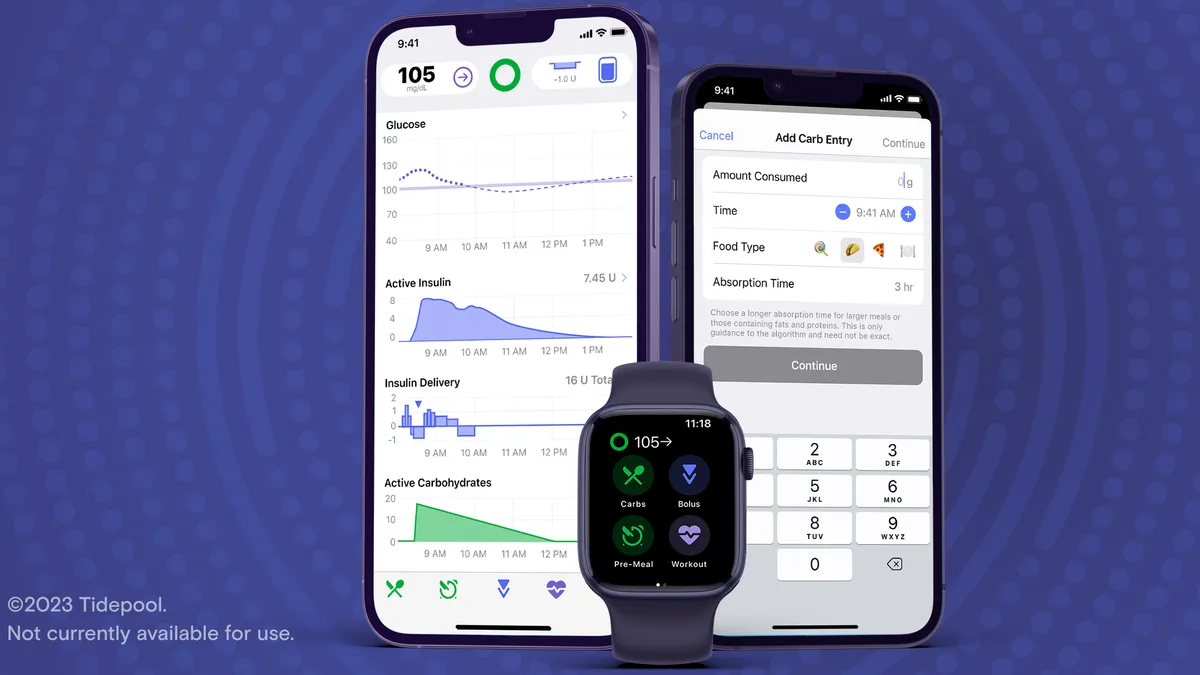
Grassroots innovation: How a patient-driven device for diabetes won FDA clearance
Tidepool Loop, an automated insulin dosing app based on one developed by the DIY diabetes community, received FDA clearance in January.
By Elise Reuter • April 3, 2023 -
Getinge’s quality woes continue: FDA categorizes another heart pump recall as Class I event
The recall is the latest in a series of compliance problems for Getinge, which has had CE marks for two products suspended.
By Nick Paul Taylor • April 3, 2023 -
Philips CEO Jakobs expects first settlement on respirators this year, Dutch newspaper says
Jakobs said he “hopes and expects” to reach a settlement with the Food and Drug Administration this year; settling suits over alleged medical damages may take longer.
By Peter Green • March 31, 2023 -
Q&A
Friday Q&A: Nevro CMO tells how the spinal device maker will defend its turf in diabetic pain relief
Dr. David Caraway says Nevro’s device stands apart from competing treatments for painful diabetic neuropathy with its clinical data and approach. The company is also launching a new product.
By Elise Reuter • March 31, 2023 -
Safety of fixed palatal expanders probed by FDA amid patient lawsuits
The investigation follows lawsuits from patients who say the device damaged their teeth and gums, and eroded their jawbones.
By Nick Paul Taylor • March 31, 2023 -
FDA qualifies tool for predicting temperature rise when orthopedic implants enter MRI scanners
Device makers can use the tool to identify femoral nail designs that would be less suitable for use in people who may undergo MRI.
By Nick Paul Taylor • March 31, 2023 -
FDA drafts guidance to ease path to updates for AI-enabled devices
The guidance describes a framework for changes to medical devices that use machine learning.
By Elise Reuter • March 30, 2023 -
Surmodics receives FDA feedback weeks after layoffs
Last month, Surmodics laid off 13% of its workforce, blaming regulatory approval delays, which also halted a $24 million milestone payment from Abbott as the firms work on a treatment for peripheral arterial disease.
By Susan Kelly • March 30, 2023 -
ICU Medical challenges GE HealthCare for Medtronic units valued at $8B: report
Medtronic wants to spin off or sell the two units to focus on faster-growing parts of its business and is talking to potential buyers.
By Nick Paul Taylor • March 30, 2023 -
FDA says it won’t issue ‘refuse to accept’ letters on cyber devices before Oct. 1
Until October 1, the FDA will work with device makers to ensure pre-market submissions have adequate cybersecurity information.
By Elise Reuter • Updated March 30, 2023 -
In a threat to diabetes tech market, scientists use implanted glucose-run fuel cell to power insulin release
If commercialized, the technology could disrupt continuous glucose monitor and insulin pump markets fought over by companies including Abbott and Medtronic.
By Nick Paul Taylor • March 29, 2023 -
Getinge heart pump has CE mark suspended by second notified body, halting sales for 3 months
TÜV SÜD took the action over concerns related to risk management, post-market surveillance and vigilance, and the timeliness of field safety corrective actions.
By Nick Paul Taylor • March 28, 2023