Medical Devices: Page 73
-
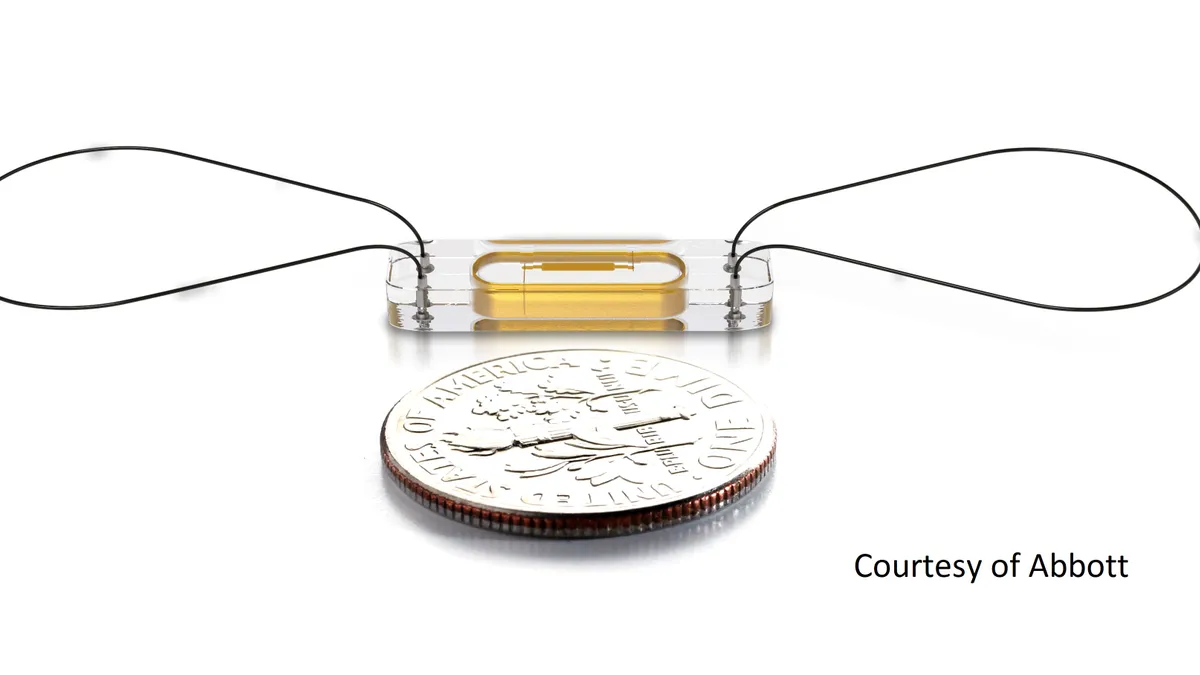
Implantable heart failure sensors cut death risk in study: Abbott
Philip Adamson, chief medical officer for Abbott’s heart failure business, called the finding “profound.”
By Susan Kelly • March 27, 2023 -
Hospitals expect procedures, capital equipment budgets to grow next year: survey
Cash-strapped hospitals say they will continue to ask medical device companies for price reductions and payment flexibilities, a survey by BTIG found.
By Elise Reuter • March 27, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Bill would require Medicare to cover breakthrough devices for four years
The bipartisan legislation, if enacted, would speed the coverage determination process for FDA-approved devices.
By Susan Kelly • March 27, 2023 -
FDA details plan to end emergency use authorizations
The agency is providing a 180-day transition period for devices that were exempted during the pandemic, and said companies that currently have an EUA should start preparing.
By Elise Reuter • March 27, 2023 -
AntennaWare secures patent for wireless tech designed to unlock implant applications
AntennaWare sees the technology facilitating deep-tissue implants that unlock new ways to monitor and treat disease.
By Nick Paul Taylor • March 27, 2023 -
Intuitive’s ecosystem means it will take J&J, Medtronic years to win surgical robot market share: analysts
The analysts expect Intuitive to retain its leadership position “even as new entrants ramp up their competitive offerings.”
By Nick Paul Taylor • March 24, 2023 -
Exactech implants may oxidize, FDA says, warning surgeons not to use any recalled joints
Exactech is recalling knee and total ankle replacements that were packaged in defective bags.
By Nick Paul Taylor • March 24, 2023 -
Medtronic merges robotic, legacy surgery businesses into one unit
The combination comes as Medtronic is going through an “aggressive transformation” intended to reduce complexity and improve capital allocation.
By Nick Paul Taylor • March 23, 2023 -
Deep Dive
Delays in reporting led FDA to late cancer warning on breast implants, advocates say
Cases need to be better tracked, and the cancer risks should be discussed with patients prior to surgery, patient advocates and physicians said.
By Elise Reuter • March 22, 2023 -
NuVasive shareholders expected to ‘begrudgingly’ back Globus’ $3.1B takeover amid lack of other bidders
Analysts said “none of the other major strategic players were in a position to bid on NuVasive — either because they were not interested or were financially tied up in other areas.”
By Nick Paul Taylor • March 22, 2023 -
Medtronic, Nvidia to combine forces on AI devices, allowing users to create new software
The companies are working together to allow physicians and developers to build AI applications for Medtronic devices, beginning with the GI Genius endoscopy platform.
By Elise Reuter • March 21, 2023 -
Spine robot maker Accelus gains another FDA clearance
The company believes its Remi device, which works with several major imaging systems, can gain traction in the market with its portable design.
By Susan Kelly • March 21, 2023 -
Boston Scientific, Medtronic expected to quickly capture new atrial fibrillation market: analysts
The companies have acquired pulsed field ablation devices in recent years, but one surgeon told analysts he prefers Boston Scientific’s device as it doesn’t require general anesthesia, leading to faster patient turnaround.
By Nick Paul Taylor • March 21, 2023 -
J&J’s Ashley McEvoy named chair of AdvaMed
McEvoy, who is J&J’s worldwide chairman of medtech, will lead the trade association’s board for the next two years.
By Elise Reuter • March 20, 2023 -
Sponsored by Quest International
How OEMs can budget aftermarket service in a recession
OEMs need to rethink how to deliver scalable, flexible service, especially in economic uncertainty.
March 20, 2023 -
Olympus accused of ‘troubling disregard for patient safety’ by FDA after flurry of warning letters
The agency’s investigation included a complaint that cracked endoscope caps caused a patient to suffer esophageal trauma.
By Nick Paul Taylor • March 20, 2023 -
FDA sets end date for raft of COVID-related shortages that began early in pandemic
Shortages of home-use ventilators and clinical sample concentrators are expected to continue.
By Nick Paul Taylor • March 20, 2023 -
Robot maker CMR Surgical appoints new CEO as it looks to ramp system sales
Supratim Bose will now lead the rollout of the company’s Versius system.
By Susan Kelly • March 17, 2023 -
Trade group warns FDA human factors proposal is ‘too expansive,’ will strain agency resources
Other groups gave a warmer welcome to the plan, with AdvaMed suggesting specific edits without criticizing the overall approach.
By Nick Paul Taylor • March 17, 2023 -
Senseonics grows sales in Q4 despite ‘softer demand’ for CGM implant in US and Germany
Demand in the U.S. was affected by “a little bit of ambiguity on pricing” ahead of a change to Medicare.
By Nick Paul Taylor • March 17, 2023 -
Medtronic wins CE mark for ablation device, invests in heart failure monitoring company
The Affera system gives physicians a choice between pulsed field ablation and radiofrequency to treat atrial fibrillation.
By Susan Kelly • March 16, 2023 -
iRhythm COO resigns amid restructuring
Douglas Devine will stay on as a consultant through 2024, as the company goes through a restructuring to become “a larger digital healthcare organization.”
By Elise Reuter • March 16, 2023 -
Tandem, targeting Medtronic and Insulet’s turf, links artificial pancreas to improved outcomes in kids
If Tandem can prove to regulators that the system can help manage young children’s diabetes, it could compete with Medtronic and Insulet, which have devices that are indicated for use in kids as young as two years.
By Nick Paul Taylor • March 16, 2023 -
Getinge buys sterilization firm Ultra Clean Systems for $16M
The Sweden-based company, which already partnered with Ultra Clean as a distributor, said the deal will “significantly expand” its offerings to sterile reprocessing departments.
By Elise Reuter • March 15, 2023 -
Joint replacement business likely to be busy for the next year or more: BTIG analysts
A backlog of patients and improved staffing at hospitals and surgery centers have the analysts projecting four to six quarters of growth for firms including Stryker and Zimmer Biomet.
By Nick Paul Taylor • March 14, 2023