Medical Devices: Page 75
-
Boston Scientific to lay off 120 people at ex-Preventice site, continuing run of medtech cuts
The job cuts are related to Boston Scientific moving some of the Texas facility’s work to a site in Minnesota.
By Nick Paul Taylor • March 1, 2023 -
ReCor follows Medtronic in making case for renal denervation as FDA mulls approval decision
An analysis of three clinical trials linked the intervention to consistent reductions in blood pressure.
By Nick Paul Taylor • March 1, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
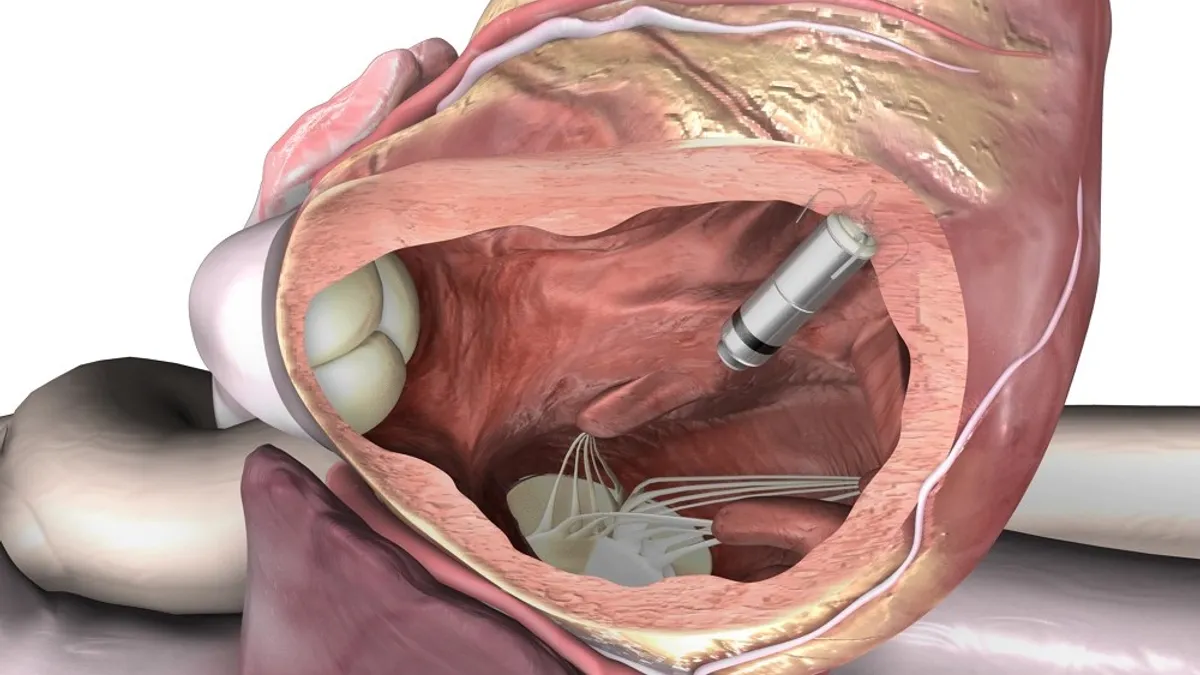
Abbott heart valve deteriorates faster than rival device, FDA says in notice
The FDA is concerned that the rate of early deterioration may be higher for Trifecta than for other surgical aortic valve replacements such as Edwards’ Magna device.
By Nick Paul Taylor • Feb. 28, 2023 -
GE HealthCare forms Chinese joint venture to sell non-premium imaging systems
The new firm will make medical equipment with a focus on primary care and rural health, putting it in competition with local manufacturers in China.
By Nick Paul Taylor • Feb. 28, 2023 -
Olympus agrees to $370M gastrointestinal stent takeover as medtech M&A rebound continues
The deal for Korea-based Taewoong, which makes stents used to help clear blockages, supports Olympus’ plan to expand in the GI market.
By Nick Paul Taylor • Feb. 28, 2023 -
European cardiologists recommend renal denervation as an adjunctive blood pressure treatment
The recommendation could open markets in Europe for the blood-pressure reduction therapy for patients who have already tried lifestyle changes and medications to treat high blood pressure.
By Elise Reuter • Feb. 27, 2023 -
Medtronic links MiniMed 780G automated insulin delivery to sustained glucose control benefits
The data indicates patients should receive automated insulin delivery earlier, rather than starting treatment with multiple daily injections, Medtronic said.
By Nick Paul Taylor • Feb. 27, 2023 -
Insulet exceeds sales forecast amid signs Omnipod 5 is winning share in diabetes pump market
The pump will be a “share taker for some time to come,” as patients switch from rival devices, said Insulet’s CFO.
By Nick Paul Taylor • Feb. 24, 2023 -
Getinge has CE marks suspended on life support sets over sterile packaging issues
Getinge said it is “aware of the severe situation this suspension puts on healthcare and critically ill patients” and is “working urgently to minimize the impact.”
By Nick Paul Taylor • Feb. 24, 2023 -
TAVR placement gets optimized with use of pumping, 3D-printed hearts by MIT researchers
The heart model predicted the hemodynamic changes in recipients of Medtronic’s Evolut R and Edwards Lifesciences’ Sapien 3.
By Nick Paul Taylor • Feb. 24, 2023 -
Tandem sees growth in new product launches after $95M loss on decline in pump placements
Craig Hallum analysts said 2022 was a “tough year” for Tandem but noted the company’s 2023 forecast should “properly” set expectations.
By Elise Reuter • Feb. 23, 2023 -
Medtronic relaunches pulmonary valve that was subject of recall
Designed to treat a congenital heart defect, the device was launched two years ago, but was recalled after a year.
By Susan Kelly • Feb. 23, 2023 -
Globus’ full-year sales top $1B as analysts continue to question wisdom of NuVasive takeover
Analysts raised concerns about Globus’ planned $3.1 billion takeover of spine specialist NuVasive, asking about the company’s “flexibility and willingness to close the deal.”
By Nick Paul Taylor • Feb. 23, 2023 -
Smith+Nephew posted underlying sales growth in Q4; profit slides
The company has made progress with product availability even as it still faces supply chain issues with semiconductors, resins and sterilization capacity.
By Nick Paul Taylor • Feb. 23, 2023 -
General Catalyst’s Elena Viboch shares medtech predictions for 2023
The venture-capital firm partner talked about the future of AI in radiology and pathology as well as the use of technology in clinical trials.
By Elise Reuter • Feb. 22, 2023 -
AliveCor says enforcement of Apple Watch import ban may take more than a year
The Biden administration upheld findings that Apple violated AliveCor’s patents, but a potential import ban rests on the outcome of a separate appeal, which could take several months.
By Elise Reuter • Feb. 22, 2023 -
Philips executives waive bonuses after year of recalls; ex-CEO gets $222,000
The waived incentives coincide with an FDA notice about the recall of some reworked ventilators that Philips initiated late last year.
By Nick Paul Taylor • Feb. 22, 2023 -
J&J loses bid for Supreme Court review of $302M vaginal-mesh verdict
The justices’ decision to let a lower court verdict stand leaves the company subject to varying state liability laws, J&J says.
By Peter Green • Feb. 21, 2023 -
Medtronic boosts full-year outlook as supply challenges subside
The medical device maker said its cardiovascular and neuroscience units helped drive quarterly revenue growth.
By Susan Kelly • Feb. 21, 2023 -
Spinal cord stimulation shows ability to improve motor function in stroke patients in small trial
The spinal implant improved strength, grip force and movement, enabling participants to recover the use of limbs incapacitated by stroke.
By Nick Paul Taylor • Feb. 21, 2023 -
Device makers gain more time to adapt to Europe’s MDR after EU vote
The European Parliament’s approval means that medtech manufacturers have until 2027 or 2028 to comply with the new rules, depending on device class.
By Susan Kelly • Feb. 17, 2023 -
Baxter spinoffs move forward as three execs made group presidents in new business structure
The appointments come as the company’s redesigned operating model takes shape ahead of a planned spinoff of its renal care and acute therapies businesses.
By Susan Kelly • Feb. 17, 2023 -
Henry Schein Q4 profit drops, hurt by falling sales of COVID test kits, PPE
Flu and COVID-19 cases increased appointment cancellations and added to staffing shortages in the quarter.
By Nick Paul Taylor • Feb. 17, 2023 -
GE’s nuclear imaging device can crush patients, FDA says, as Class I recall issued
GE HealthCare has told customers to stop using the machines until company technicians can inspect them and replace dangerous parts.
By Nick Paul Taylor • Feb. 17, 2023 -
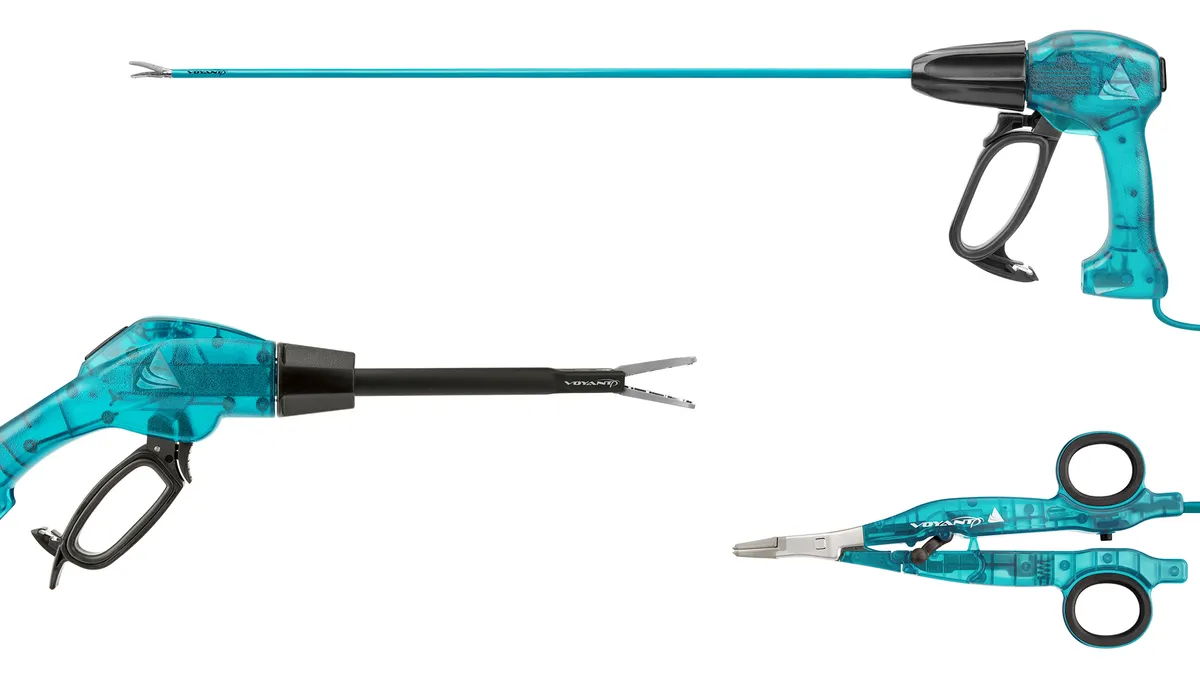
Applied Medical suit against Medtronic alleges surgical-device bundling practice curbs competition
Applied says in its filing that Medtronic “has conspired” to bundle devices for cutting tissue and sealing vessels “in a way that is unhealthy for competition.”
By Nick Paul Taylor • Updated Feb. 16, 2023