Medical Devices: Page 89
-
Medtronic gets third Class I label in 2022 for latest HVAD recall
The latest recall is for nearly 40,000 batteries for the HeartWare Ventricular Assist Device system.
By Ricky Zipp • Aug. 19, 2022 -
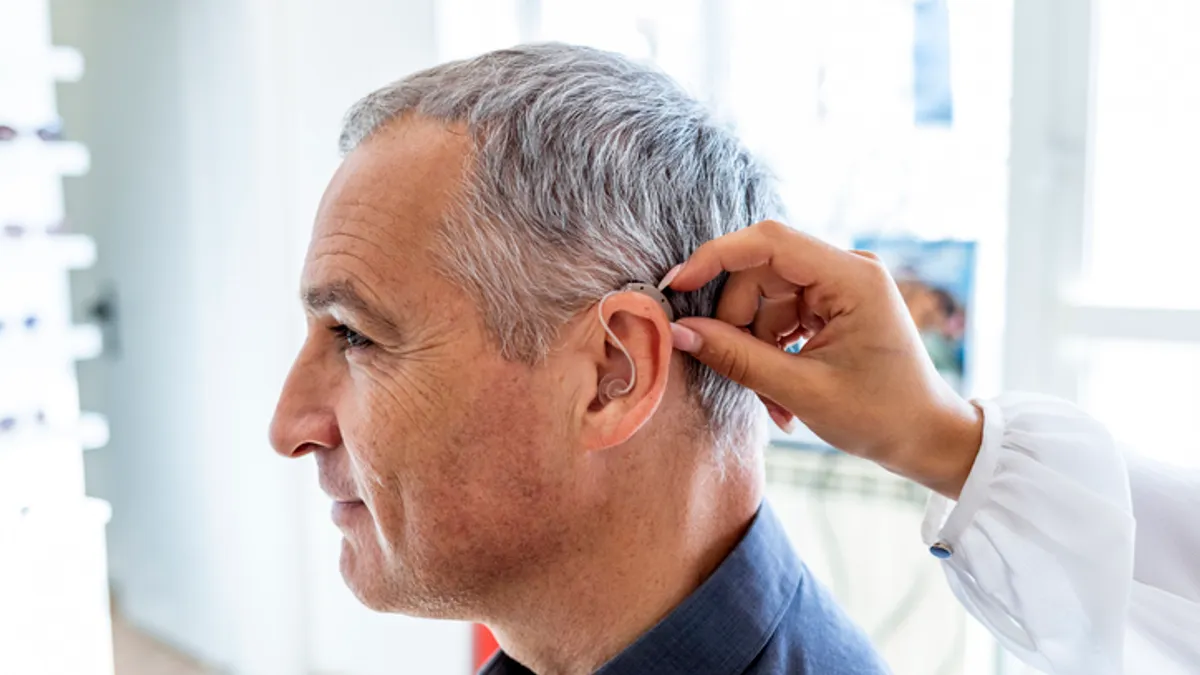
Eargo plans to sell its hearing aids in stores after over-the-counter ruling
Best Buy, which announced it would sell over-the-counter hearing aids in more than 300 stores, has listed the Eargo 6 as one of the products it will offer.
By Elise Reuter • Aug. 18, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medical device recalls hit two-year high in Q2, report finds
While the number of recalls jumped in the period, the total units affected dropped to the lowest point in five years.
By Ricky Zipp • Aug. 18, 2022 -
New entrants may shake up hearing-aid market after FDA clears path for OTC sales
Startups, large tech companies and even traditional hearing-aid manufacturers are expected to offer over-the-counter devices.
By Elise Reuter • Aug. 17, 2022 -
Moderna, after firing newly hired CFO, finds replacement in PerkinElmer executive
The COVID-19 vaccine maker has appointed James Mock, currently PerkinElmer’s CFO, as its next finance head, three months after ousting Jorge Gomez from the role.
By Ned Pagliarulo • Aug. 17, 2022 -
Philips’ next CEO, Roy Jakobs, provides ‘continuity’ amid ongoing recall: Barclays
Roy Jakobs currently leads the company’s connected care business and has helped oversee its recall response.
By Ricky Zipp • Aug. 17, 2022 -
Visibly wins FDA clearance for self-administered online vision test
The agency has placed restrictions on the test, limiting it to adults aged 22 to 40 years and cautioning that it only gives “supportive recommendations.”
By Nick Paul Taylor • Aug. 17, 2022 -
FDA reviews rise in Philips respiratory device reports, including 44 deaths
The agency received more than twice as many reports linked to foam breakdown from May to July than from April 2021 to April 2022.
By Nick Paul Taylor • Aug. 17, 2022 -
Outset lands VA contract for portable hemodialysis system in ‘modest’ boost for sales
Stifel analysts said the contract could generate additional sales of as much as $50 million over five years.
By Nick Paul Taylor • Aug. 16, 2022 -
Hearing aids to be available over the counter following final FDA rule
Hearing aids were introduced for over-the-counter purchase in mid-October
By Elise Reuter • Aug. 16, 2022 -
Philips CEO Frans van Houten to step down in October amid recall woes; Jakobs named successor
The leadership change follows a July announcement that the company is in talks on a consent decree with the Department of Justice.
By Ricky Zipp • Aug. 16, 2022 -
Smartphone-linked blood pressure monitors fail to beat traditional devices, study finds
Individuals have been able to check their blood pressure at home using devices that lack connectivity features for years, raising the question of whether the technology improves outcomes.
By Nick Paul Taylor • Aug. 16, 2022 -
Boston Scientific acquires biomaterials firm Obsidio to bolster embolization business
Obsidio’s Gel Emobolic Material technology was cleared by the FDA in July.
By Ricky Zipp • Aug. 15, 2022 -
J&J, other orthopedics companies faced unequal pandemic recovery in second quarter
As procedure volumes increased, some businesses such as Zimmer Biomet gained share while others lagged.
By Elise Reuter • Aug. 15, 2022 -
BD’s recall of emergency vascular access devices labeled Class I event by FDA
The company first announced the recall in June. There have been no reports of serious injuries or deaths tied to the issue.
By Nick Paul Taylor • Aug. 15, 2022 -
Abbott will invest $450M in Ireland to expand EU production of FreeStyle Libre CGMs
The move, which will give Abbott a new facility in Kilkenny and enhance its existing site in Donegal, comes amid stiffening competition with Dexcom.
By Nick Paul Taylor • Aug. 15, 2022 -
ResMed CEO Farrell says supply chain ‘very much in flux’ amid signs of improvement
Mick Farrell told investors on an earnings call Thursday that one improvement has been the supply of semiconductor chips. Still, the executive stressed the importance of prioritizing medical devices.
By Ricky Zipp • Aug. 12, 2022 -
Abbott blood test predicts death, unfavorable outcomes in traumatic brain injury patients, study finds
Abbott is pursuing FDA clearance for the breakthrough-designated TBI test to expand access to a diagnostic that predicted death and severe disability.
By Nick Paul Taylor • Aug. 12, 2022 -
Q&A
Friday Q&A: Francine Kaufman, Senseonics’ CMO, talks growing users, a 365-day CGM, doctor recruitment
Kaufman spoke about Senseonics’ release of its 180-day CGM in the U.S., developing a unique product in the diabetes technology market and competing with Dexcom and Abbott.
By Ricky Zipp • Aug. 12, 2022 -
‘Underfunded’ FDA falls short in ensuring medical devices protect against cyberattacks, experts say
Medical device manufacturers argue the agency’s current rules on cybersecurity requirements are too restrictive and should be phased in gradually.
By Elise Reuter • Aug. 11, 2022 -
Insulet raises forecast as Omnipod 5 sales climb; Tandem expects tougher market in second half
Both insulin pump companies grew revenue by more than 10% in the second quarter, but Tandem lowered its 2022 forecast amid a tougher market, while Insulet increased its guidance.
By Ricky Zipp • Aug. 11, 2022 -
Medtronic issues Class I recall of implantable cardioverter defibrillators in U.S.
The devices may deliver a reduced shock during high-voltage therapy, about 79% of the programmed energy.
By Nick Paul Taylor • Aug. 11, 2022 -
Roundup: Medical device industry confident it can ride out recession, wary of slowing capital sales
While companies may weather a recession, they still face challenges like hospital staffing shortages, supply chain disruption and inflation, RBC Capital Markets analysts wrote.
By Nick Paul Taylor • Updated Aug. 10, 2022 -
Boston Scientific says it’s investigating allegations of anti-bribery law violations
The company says the issue is centered on its operations in Vietnam.
By Nick Paul Taylor • Aug. 10, 2022 -
Eargo’s Q2 revenue slides amid decline in hearing-aid shipments
The company’s gross system shipments fell by more than 8,000 when compared to the year-earlier period.
By Ricky Zipp • Aug. 9, 2022