Medical Devices: Page 88
-
Invacare drops CEO, changes board amid broader restructuring
With seven of its eight directors independent of management under an accord with its largest investor, the company is working to rebound from supply chain problems.
By Elise Reuter • Aug. 31, 2022 -
Jury orders BD unit to pay $4.8M to patient in latest hernia mesh case
The award comes amid a string of defeats for major medical device makers over alleged injuries from surgical mesh products.
By Ricky Zipp • Aug. 31, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Philips settles government claim for $4.2M over military certification of portable patient monitors
Philips conceded that it had failed to recertify the MP2 patient monitor for safe use in airplanes after modifying the devices it was supplying to the U.S. Army and Air Force.
By Elise Reuter • Aug. 31, 2022 -

 Retrieved from Hamilton website on August 30, 2022
Retrieved from Hamilton website on August 30, 2022
Recall Watch: Hamilton ventilator, Intera pump recalls get Class I label from FDA
The FDA assigned the recalls to its most serious risk category in light of the potential for serious injuries and deaths.
By Nick Paul Taylor • Aug. 30, 2022 -
 Retrieved from Olympus on August 30, 2022
Retrieved from Olympus on August 30, 2022
Olympus to sell microscope unit for $3.1B as it bolsters focus on medical technology
Bain Capital has taken the other side of the deal, paying $3.1 billion for a business it said is “at the frontier of digital optical technology in life sciences and industrial end markets.”
By Nick Paul Taylor • Aug. 30, 2022 -
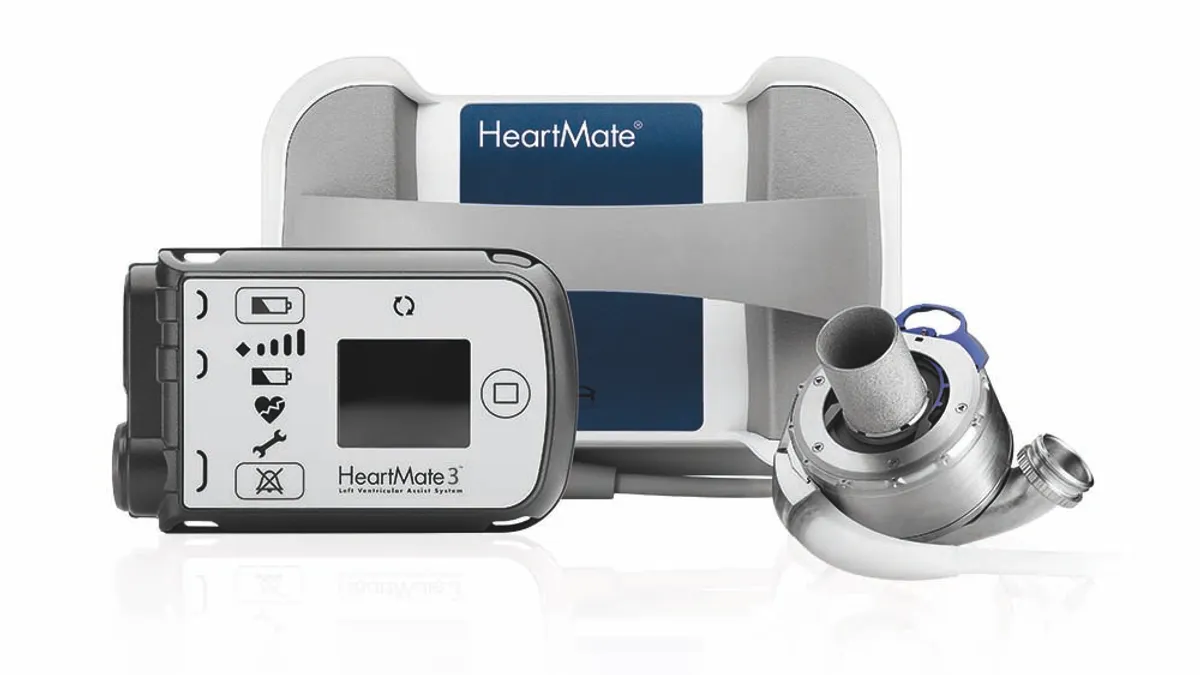
Abbott HeartMate 3 LVAD shows improved survival rate compared to older heart pump
After five years, Abbott found that patients implanted with the HeartMate 3 LVAD had an improved survival rate compared to those implanted with the HeartMate II.
By Elise Reuter • Aug. 29, 2022 -
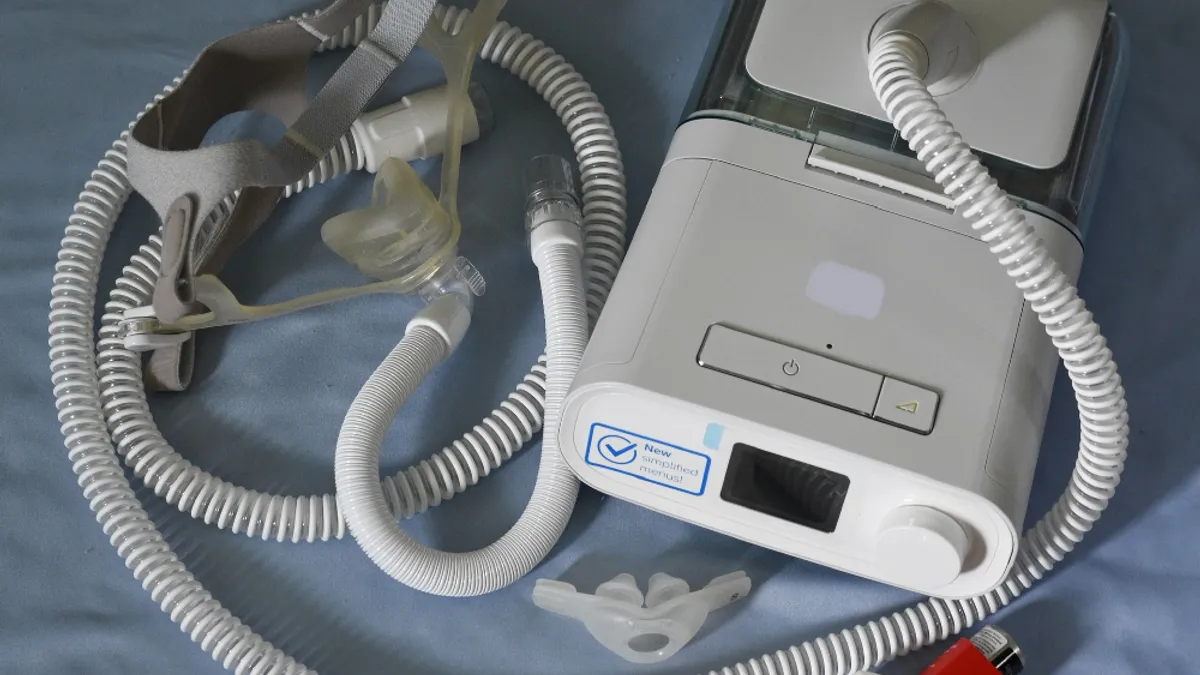
Philips recalls some BiPAP machines because of contaminated plastic
The FDA said the latest action is not associated with the company’s sleep apnea machine recall, although some devices are affected by both.
By Ricky Zipp • Aug. 29, 2022 -
Medtronic’s extravascular ICD meets safety, efficacy endpoints in recent study
The data could give Medtronic a competitor to Boston Scientific’s subcutaneous implantable defibrillator system.
By Elise Reuter • Aug. 29, 2022 -
FDA takes N95 respirators off medical device shortage list
Respirators were one of the first medical devices identified as being in critical shortage during the COVID-19 public health emergency, the agency said.
By Ricky Zipp • Aug. 29, 2022 -
Q&A
Akili CEO Martucci sees ‘massive’ need for company’s ADHD video-game treatment
With enough cash now to last two years, the company hopes it can change the way children with ADHD are treated, offering an alternative to pharmaceuticals.
By Elise Reuter • Aug. 29, 2022 -

 Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
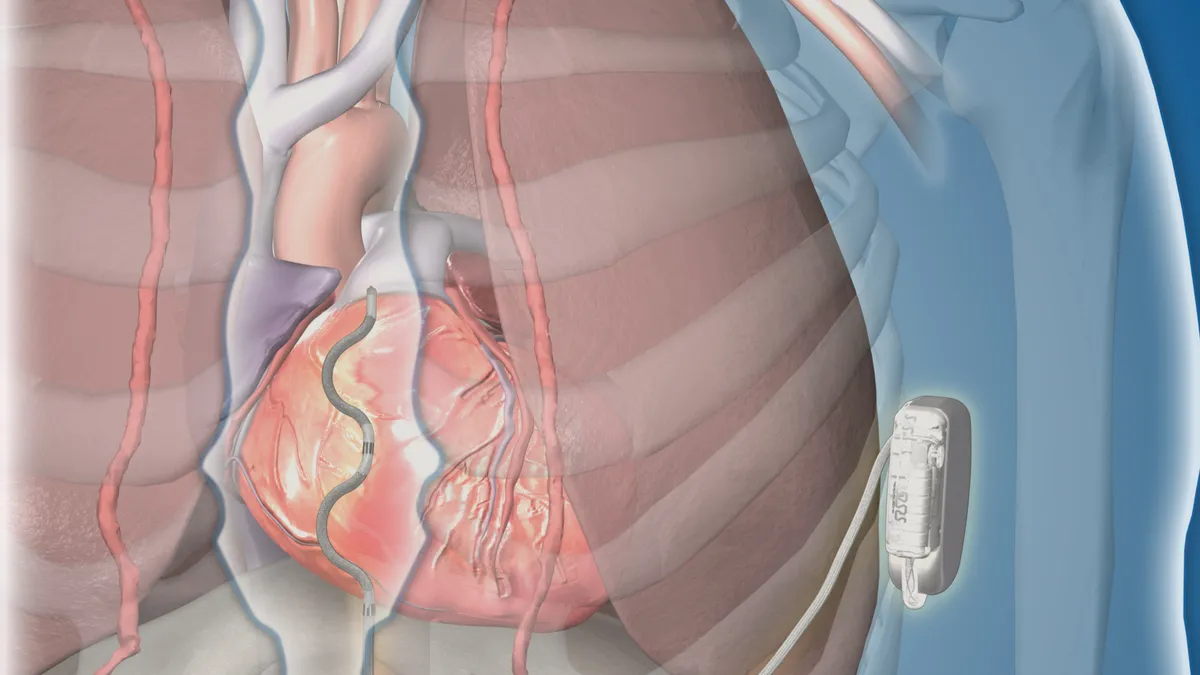
Medtronic reports six more injuries, one additional death related to HVAD battery problems
The company pulled the heart pump from the market last year, after the FDA received word of more than 3,000 deaths linked to various problems with the device, according to published reports.
By Elise Reuter • Aug. 26, 2022 -
Penumbra’s catheters achieve first-pass revascularization in 68.9% of stroke patients
A separate study found the device cleared clots in patients with tandem lesions, meeting the criteria for successful restoration of blood flow in 84% of subjects.
By Nick Paul Taylor • Aug. 26, 2022 -
Review finds ‘paucity of robust evidence’ on impact of AI clinical outcomes
Of the nearly 12,000 articles on artificial intelligence, only 39 described randomized controlled trials, making it difficult to quantify the clinical benefits of using AI-assisted tools for patient care.
By Nick Paul Taylor • Aug. 26, 2022 -
Medtronic gets CE mark for drug-eluting stent and kicks off European launch
Medtronic calculated that changes to the stent resulted in a 16% improvement in drug deliverability.
By Nick Paul Taylor • Aug. 25, 2022 -
 Retrieved from Integra Life on August 24, 2022
Retrieved from Integra Life on August 24, 2022
Integra pulls intracranial pressure monitors for inaccurate readings
After announcing an immediate voluntary removal of the devices, Integra trimmed its sales forecast for the quarter.
By Elise Reuter • Aug. 24, 2022 -

 Retrieved from Abbott.com on August 24, 2022
Retrieved from Abbott.com on August 24, 2022
Abbott wins FDA approval for spinal cord stimulation treatment of multi-site pain
The approval gives Abbott a new angle as it seeks to win market share from Boston Scientific, Medtronic and Nevro.
By Nick Paul Taylor • Aug. 24, 2022 -
 Courtesy of https://news.getinge.com/hs-fs/hubfs/CUsersu4003864DesktopServo-u%204.0-1.jpg?width=1800&name=CUsersu4003864DesktopServo-u%204.0-1.jpg
Courtesy of https://news.getinge.com/hs-fs/hubfs/CUsersu4003864DesktopServo-u%204.0-1.jpg?width=1800&name=CUsersu4003864DesktopServo-u%204.0-1.jpg
Getinge’s recall of 11,000 ventilators labeled Class I event by FDA
The company is fixing a software bug that can inadvertently stop the ventilator from working.
By Nick Paul Taylor • Aug. 24, 2022 -
Philips respirator lawsuit builds as plaintiffs’ attorneys outline lengthy recall delays
New filings in a class action lawsuit allege that Philips waited years to disclose the risk of foam used in its sleep apnea machines and respirators, causing illness and injuries.
By Elise Reuter , Peter Green • Updated Aug. 23, 2022 -
Medtronic CEO says supply chain starting to stabilize as sales dropped, profits rose in Q1
The company is keeping its revenue forecast for fiscal year 2023 as it works to resolve issues with packaging and resins.
By Elise Reuter • Aug. 23, 2022 -
Insulet’s Omnipod 5 insulin delivery system approved for preschool children
Adding preschoolers will broaden the market for the Omnipod after the launch of the device for people aged six years and older helped Insulet boost U.S. sales by 31% in the second quarter.
By Nick Paul Taylor • Aug. 23, 2022 -
Teleflex will buy Standard Bariatrics for $170M to gain Titan stapler
Standard’s investors could receive an additional $130 million if the unit reaches certain commercial milestones under Teleflex’s ownership.
By Elise Reuter • Aug. 22, 2022 -
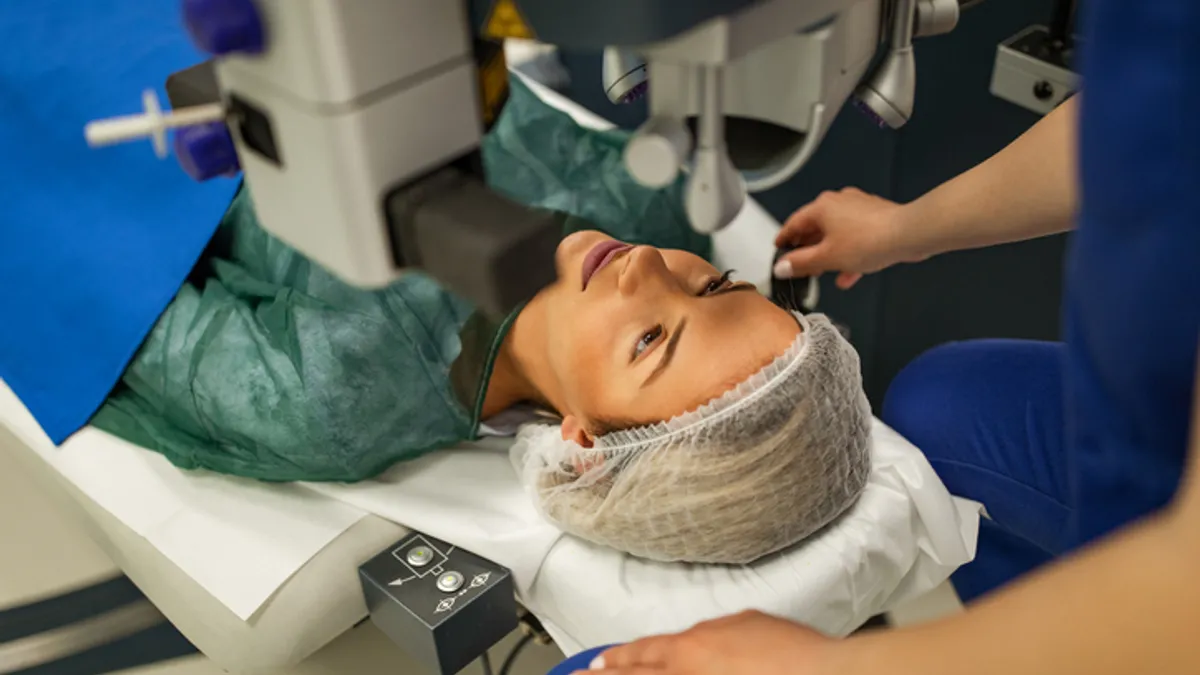
Laser vision correction procedures fell 16% in the second quarter
The slowdown raises questions about whether demand is waning or if there was a pull-through in procedures last year.
By Elise Reuter • Aug. 22, 2022 -
Medical device industry poised to withstand an economic slowdown, experts say
While procedures volumes may decline, analysts said the effects likely won’t reach levels seen in 2020 or during the Great Recession.
By Ricky Zipp • Aug. 22, 2022 -
Edwards gets CE mark for Pascal Precision system, allowing device to be marketed in Europe
The system, which is used to treat patients with mitral or tricuspid regurgitation, is also expected to get FDA approval by the end of the year.
By Elise Reuter • Aug. 19, 2022 -
Q&A
Friday Q&A: ResMed CEO Mick Farrell discusses Philips recall, improving supply chain, prioritizing patients
The executive spoke about managing a 12-month patient backlog following a spike in demand due to Philips’ recall and supply constraints.
By Ricky Zipp • Aug. 19, 2022