FDA
-
HHS elevates officials into Kennedy’s inner circle in advance of midterms
The HHS secretary has four new senior advisors to help him “move faster and go further” on his Make America Healthy Again agenda. The appointments preempt the GOP’s midterm messaging push.
By Rebecca Pifer Parduhn • Feb. 13, 2026 -
Novocure wins FDA approval to treat pancreatic cancer with electric fields
Winning approval to target the 15,000 U.S. patients with locally advanced pancreatic cancer is the first step in a broader expansion in the tumor type.
By Nick Paul Taylor • Feb. 13, 2026 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA breakthrough program starts FY2026 at steady pace
Orthopedics was the most active area over the second half of 2025, with the FDA issuing 13 breakthrough designations to devices in the therapeutic area.
By Nick Paul Taylor • Feb. 9, 2026 -
J&J recalls coil systems used in aneurysm treatment
Healthcare providers are advised to stop using the devices after a failure to detach was linked with four serious injuries and one death.
By Susan Kelly • Feb. 6, 2026 -
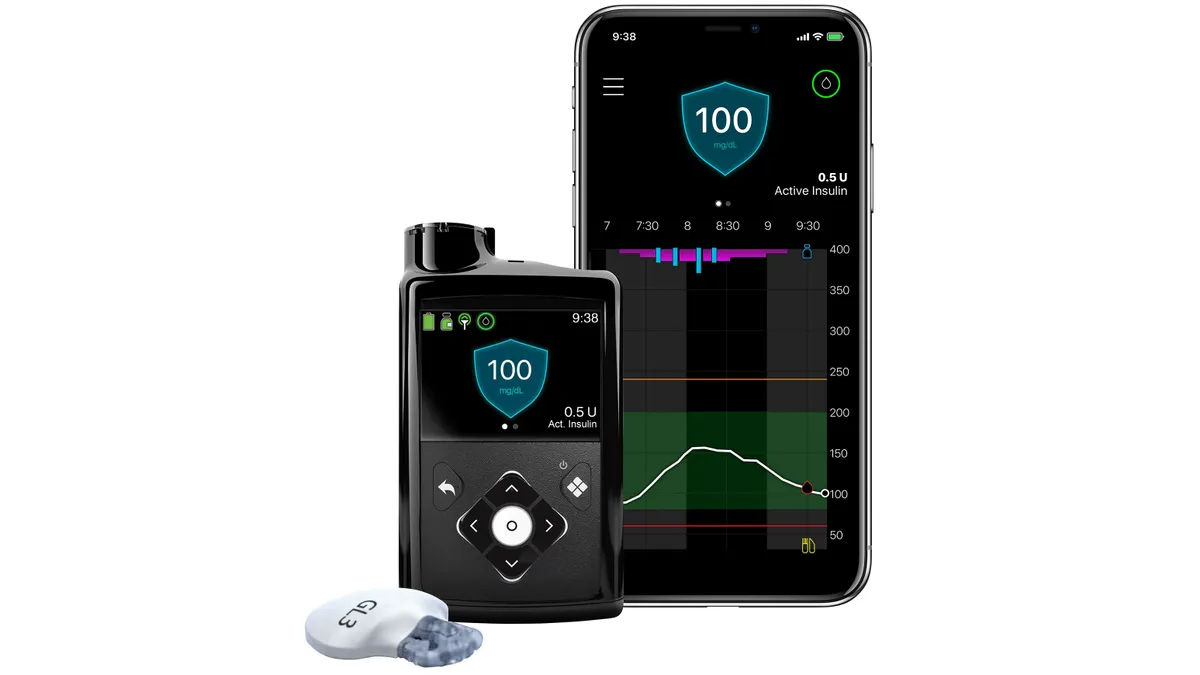
Medtronic wins expanded FDA label for 780G insulin pump
The 510(k) decision clears the device for use with ultra-rapid-acting insulins from Eli Lilly and Novo Nordisk.
By Nick Paul Taylor • Feb. 5, 2026 -
J&J corrects Impella heart pumps over issue linked to 22 serious injuries
The urgent correction notice is the latest in a series of actions related to the safety of Impella devices.
By Nick Paul Taylor • Feb. 4, 2026 -
Beta Bionics receives FDA warning letter
The letter, which the FDA has not yet published, does not affect Beta Bionics’ ability to market, manufacture or distribute products, the company said.
By Elise Reuter • Feb. 2, 2026 -
Grail files for FDA approval of multi-cancer early detection test
Premarket approval could help the company secure coverage from commercial payers and Medicare.
By Nick Paul Taylor • Feb. 2, 2026 -
Top medtech trends to watch in 2026
From M&A to surgical robotics and user fee negotiations, the medical device industry has a busy year ahead. Check out MedTech Dive’s roundup of the top medtech trends to watch in 2026.
By Ricky Zipp • Jan. 29, 2026 -
CDRH on track for review timelines despite staff cuts
The Center for Devices and Radiological Health said it is on track to meet review times specified by user fee agreements, despite “resource challenges.”
By Elise Reuter • Jan. 29, 2026 -
FDA guidance eases wearables oversight. But experts have questions about what’s next.
Experts say two new guidances — issued without the usual public comment period — leave questions about how patients will navigate a growing pool of wearables.
By Elise Reuter • Jan. 27, 2026 -
Integra recalls wound and burn devices amid reports of serious injuries
Packaging failures could lead to breaches in the sterile barrier and patient infections.
By Nick Paul Taylor • Jan. 23, 2026 -
AdvaMed leaders on tariffs, MDUFA and wearables
AdvaMed CEO Scott Whitaker and Mick Farrell, new board chair of the trade group, weighed in on important issues for the medtech industry one year into the Trump administration.
By Elise Reuter • Jan. 22, 2026 -
Boston Scientific recalls stent over issue linked to 3 deaths
The company said the deaths were associated with off-label or investigational use of the device.
By Nick Paul Taylor • Jan. 20, 2026 -
Deep Dive
5 topics to watch as MDUFA negotiations restart
The next Medical Device User Fee Amendments, which will set how much the FDA can raise from the industry in fees over a five-year period, are expected to go to Congress this year.
By Elise Reuter • Jan. 14, 2026 -
FDA exempts more wearable, AI features from oversight
In a pair of guidance documents released Tuesday, the regulator clarified the types of wellness features and clinical decision support tools that don’t fall under medical device oversight.
By Elise Reuter • Jan. 8, 2026 -
Deep Dive
4 medtech topics to watch in 2026
From insurance coverage questions to M&A and tariffs, here are the top storylines to watch in the medical device space in 2026.
By Ricky Zipp • Jan. 8, 2026 -
Stereotaxis wins FDA approval for robotically navigated ablation catheter
Stereotaxis’ CEO said the company has been “hampered clinically, commercially and strategically” by its prior dependence on a J&J catheter.
By Nick Paul Taylor • Jan. 7, 2026 -
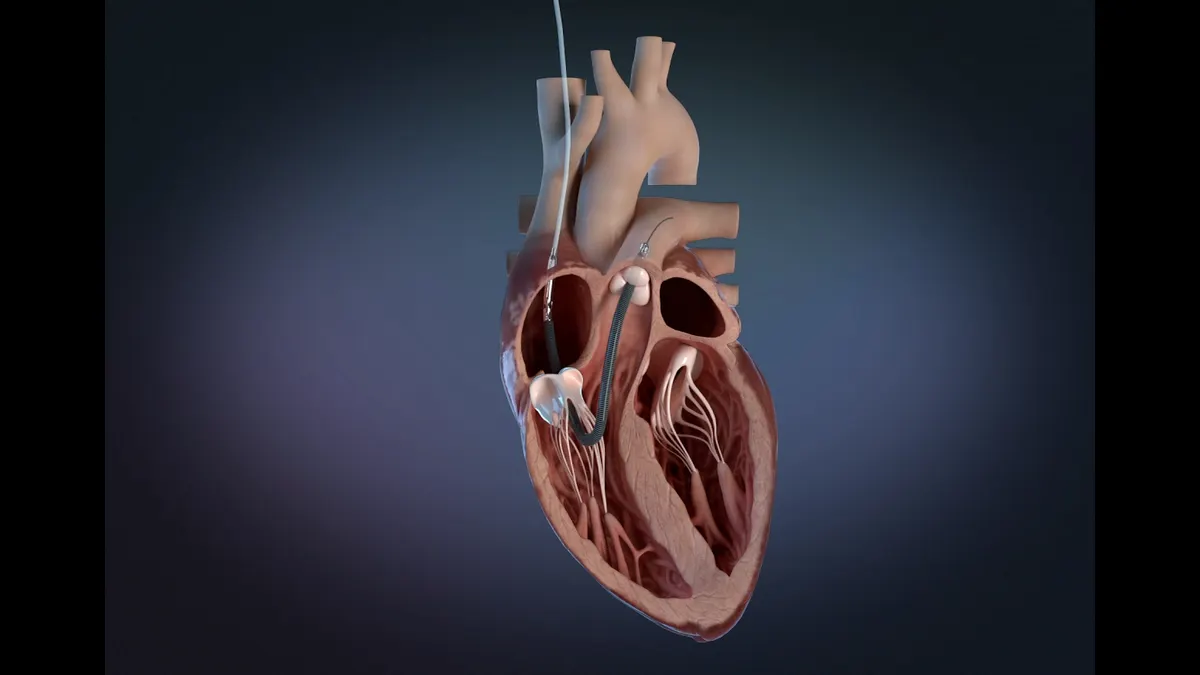
Edwards receives FDA approval for mitral valve replacement system
The Sapien M3 device is the first approved mitral regurgitation treatment to use a transseptal approach, Edwards said.
By Elise Reuter • Dec. 23, 2025 -
FDA posts Class I recall notice about Medtronic heart vent catheters
The company received three complaints about patients who had perforation injuries linked to the devices.
By Nick Paul Taylor • Dec. 23, 2025 -
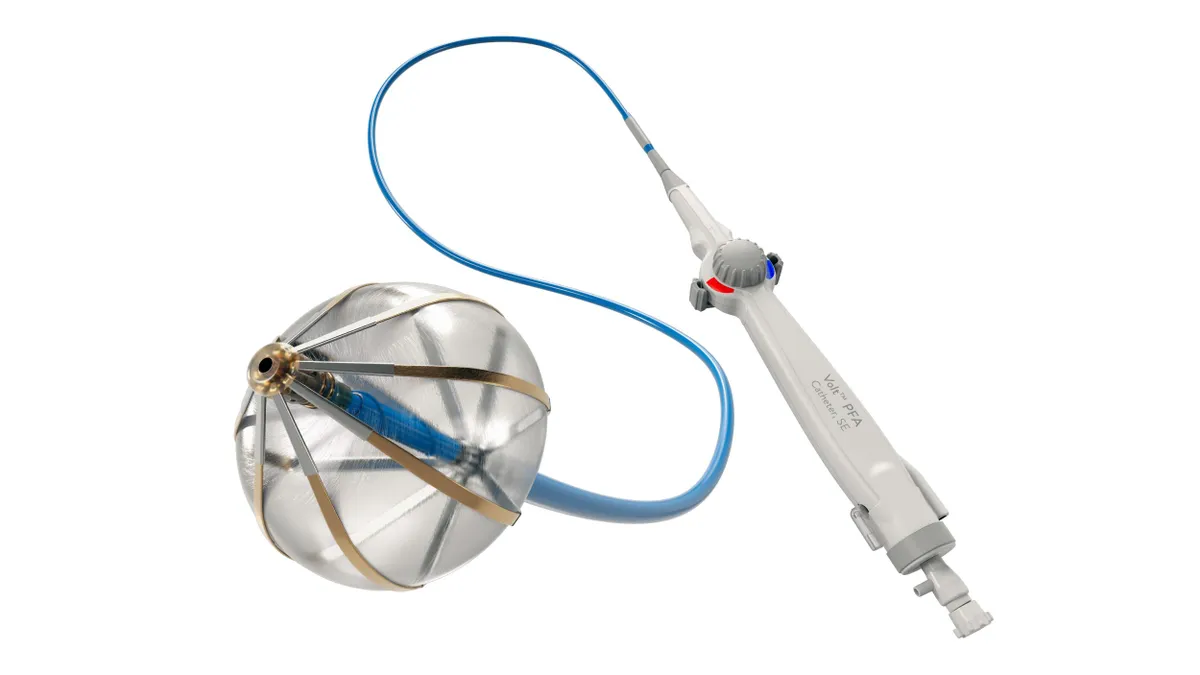
Abbott receives FDA approval for Volt PFA system
Abbott can now compete with Medtronic, Boston Scientific and J&J in the U.S. for the fast-growing pulsed field ablation market.
By Elise Reuter • Dec. 22, 2025 -
Abbott receives clearance for heart delivery device in premature babies
Physician feedback has informed a delivery device designed to make procedures safer and easier.
By Nick Paul Taylor • Dec. 22, 2025 -
FDA gets mixed feedback on performance monitoring for AI
Medtech industry groups said the FDA should use existing regulatory and quality tools to monitor performance, while medical groups said device manufacturers should be responsible for monitoring AI.
By Elise Reuter • Dec. 16, 2025 -
FDA needs more staff, authority to oversee device recalls, watchdog finds
“FDA's challenges — such as insufficient staffing — can create inefficiencies in the process and potentially put lives at risk,” the U.S. Government Accountability Office said.
By Elise Reuter • Dec. 15, 2025 -
FDA pilots allowing digital health devices access to CMS payment program
Companies can ask the FDA to waive premarket authorization and investigational device requirements while they collect real-world data in a CMS program.
By Nick Paul Taylor • Dec. 10, 2025