FDA: Page 2
-
FDA gets mixed feedback on performance monitoring for AI
Medtech industry groups said the FDA should use existing regulatory and quality tools to monitor performance, while medical groups said device manufacturers should be responsible for monitoring AI.
By Elise Reuter • Dec. 16, 2025 -
FDA needs more staff, authority to oversee device recalls, watchdog finds
“FDA's challenges — such as insufficient staffing — can create inefficiencies in the process and potentially put lives at risk,” the U.S. Government Accountability Office said.
By Elise Reuter • Dec. 15, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Courtesy of Intuitive Surgical
Courtesy of Intuitive Surgical Trendline
TrendlineTop 5 stories from MedTech Dive
From the top medtech trends to watch in 2026 to haphazard layoffs at the Food and Drug Administration and the evolving use of AI in the medtech sector, here is a collection of top stories from MedTech Dive.
By MedTech Dive staff -
FDA pilots allowing digital health devices access to CMS payment program
Companies can ask the FDA to waive premarket authorization and investigational device requirements while they collect real-world data in a CMS program.
By Nick Paul Taylor • Dec. 10, 2025 -
Deep Dive
What medtech firms can learn from Whoop’s warning letter
An FDA warning letter about the wearable company’s blood pressure feature raises questions about the blurring lines between medtech and wellness.
By Elise Reuter • Dec. 8, 2025 -
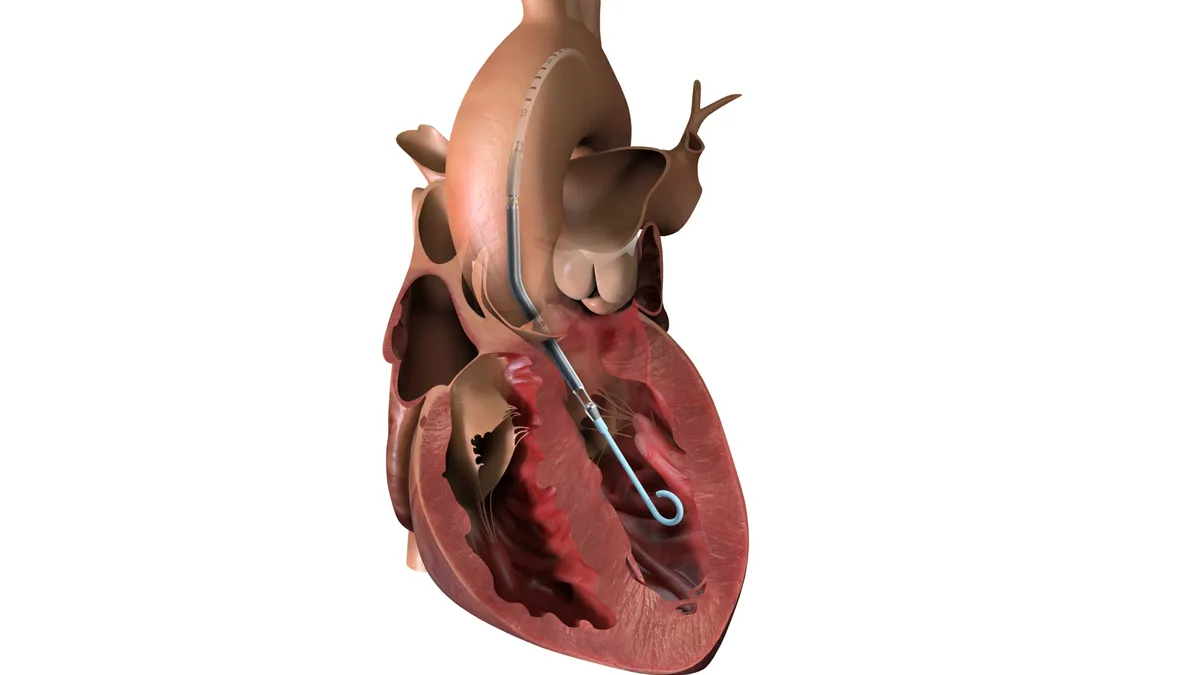
FDA advisory panel votes unanimously against J&J heart shunt
Panelists focused on study data that showed the implant did not improve outcomes in heart failure patients.
By Susan Kelly • Dec. 5, 2025 -
Olympus reinforces advice over device issue linked to 113 serious injuries
The company received complaints that the ligation loop can become unintentionally anchored in place.
By Nick Paul Taylor • Dec. 4, 2025 -
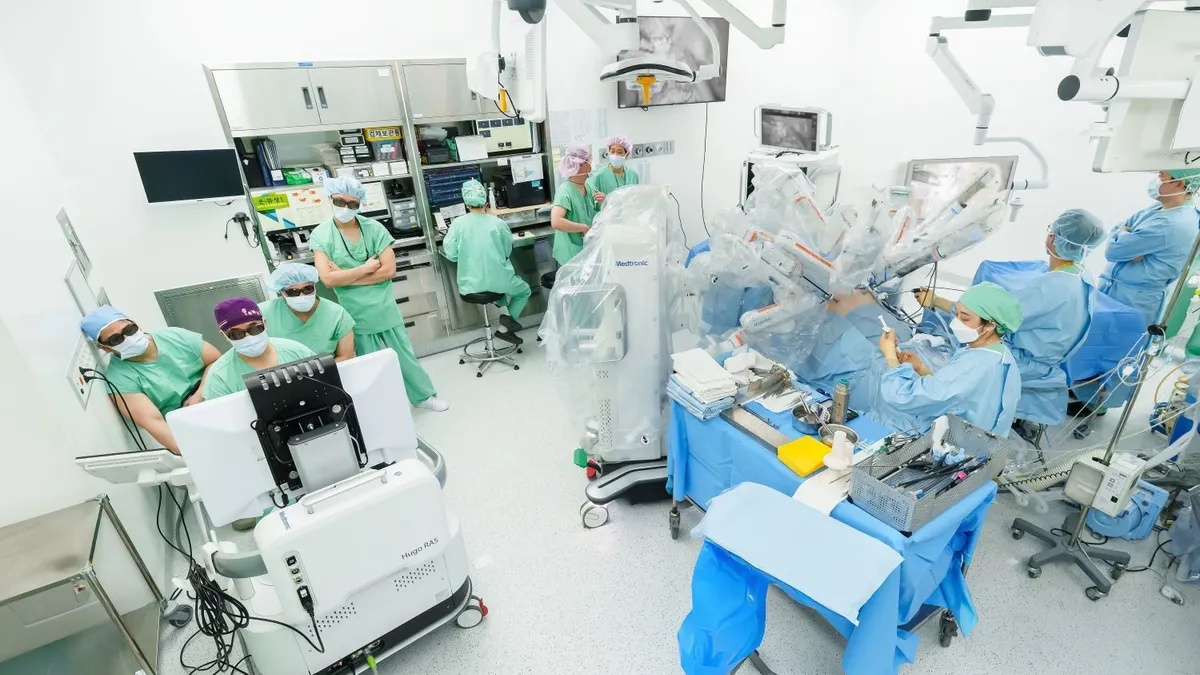
Medtronic’s Hugo surgical robot earns FDA clearance
With the U.S. authorization for urologic procedures, “there is now choice” for hospitals that want to expand their robotic programs, a company executive said.
By Susan Kelly • Dec. 4, 2025 -
Richard Pazdur, FDA drug czar, to retire from agency
Weeks after being named head of CDER, Pazdur has submitted paperwork to step down at the end of the month, exacerbating what’s already been a turbulent year at the FDA.
By Ben Fidler • Dec. 2, 2025 -
Cleveland Diagnostics wins FDA nod for prostate cancer test
The IsoPSA test is intended to help assess whether patients with elevated PSA levels should go on to receive a biopsy procedure.
By Susan Kelly • Dec. 2, 2025 -
FDA flags risk from dropped BD Alaris pumps after 2 injury reports
BD told customers to immediately remove from use any devices they drop or severely jar.
By Nick Paul Taylor • Dec. 2, 2025 -
Ceribell wins FDA clearance for seizure-detection algorithm in neonates
By acquiring new accounts and expanding into NICUs managed by existing clients, Ceribell calculates it can unlock a $400 million market.
By Nick Paul Taylor • Nov. 25, 2025 -
Zimmer wins FDA clearance for updated knee robot
Competing with companies including Stryker and J&J, Zimmer has moved to strengthen its position by developing an updated robot.
By Nick Paul Taylor • Nov. 17, 2025 -
Stereotaxis nets FDA clearance for redesigned surgical robot
The company also posted weaker third-quarter results, but CEO David Fischel said orders for the new GenesisX system, when fully launched, would outpace the rate for an older model.
By Susan Kelly • Nov. 12, 2025 -
Zimmer wins FDA’s breakthrough status for tech to prevent joint infections
Integrating a controlled-release iodine layer into hip implants could reduce the rate of potentially fatal infections.
By Nick Paul Taylor • Oct. 30, 2025 -
Olympus execs talk about import alert, GI robot and AI
CEO Bob White and Chief Strategy Officer Gabriela Kaynor discussed the medtech company’s quality and technology priorities after a year of change.
By Elise Reuter • Oct. 28, 2025 -
Top RFK aide lashes out against healthcare industry for profiting off of illness
Calley Means stopped short of accusing hospitals, insurers and drug companies from actively working to keep Americans sick, but said it was an "economic fact" that the companies benefit financially when people are ill.
By Susanna Vogel • Oct. 23, 2025 -
HHS says it can still fire workers despite judge’s order pausing shutdown layoffs
The 982 HHS employees who received reduction-in-force notices are not represented by the unions that are plaintiffs in the case, the department’s head of personnel argued in a court filing.
By Rebecca Pifer Parduhn • Oct. 20, 2025 -
Cybersecurity triggers another recall of J&J’s Impella heart pump controller
While no patients have been harmed, the FDA categorized the action as a Class I recall because of the potential for serious injury or death.
By Nick Paul Taylor • Oct. 14, 2025 -
Trump administration moves to fire HHS employees amid shutdown
Upwards of 1,000 HHS employees were affected. However, the department has rolled back some of the layoffs at the CDC, where the brunt of the terminations were concentrated.
By Rebecca Pifer Parduhn • Updated Oct. 14, 2025 -
Intuitive’s challengers continue to advance
Companies creating new robotic platforms marked additional milestones heading into the fourth quarter. Catch up with their progress in this roundup of the latest developments in surgical robotics.
By Susan Kelly • Oct. 10, 2025 -
FDA’s presence at AdvaMed conference shrinks amid shutdown
Michelle Tarver, director of the Center for Devices and Radiological Health, and other center officials attended virtually as a government shutdown continues.
By Elise Reuter • Oct. 9, 2025 -
AdvaMed CEO: ‘Just give us some certainty’
At AdvaMed’s annual conference, CEO Scott Whitaker addressed policy issues around tariffs, FDA staff cuts and the government shutdown.
By Elise Reuter • Oct. 8, 2025 -
CDRH shares regulatory guidance priorities for the coming 12 months
Use of real-world evidence to support regulatory decisions and predetermined change control plans for medical devices are two areas of focus for the agency.
By Nick Paul Taylor • Oct. 2, 2025 -
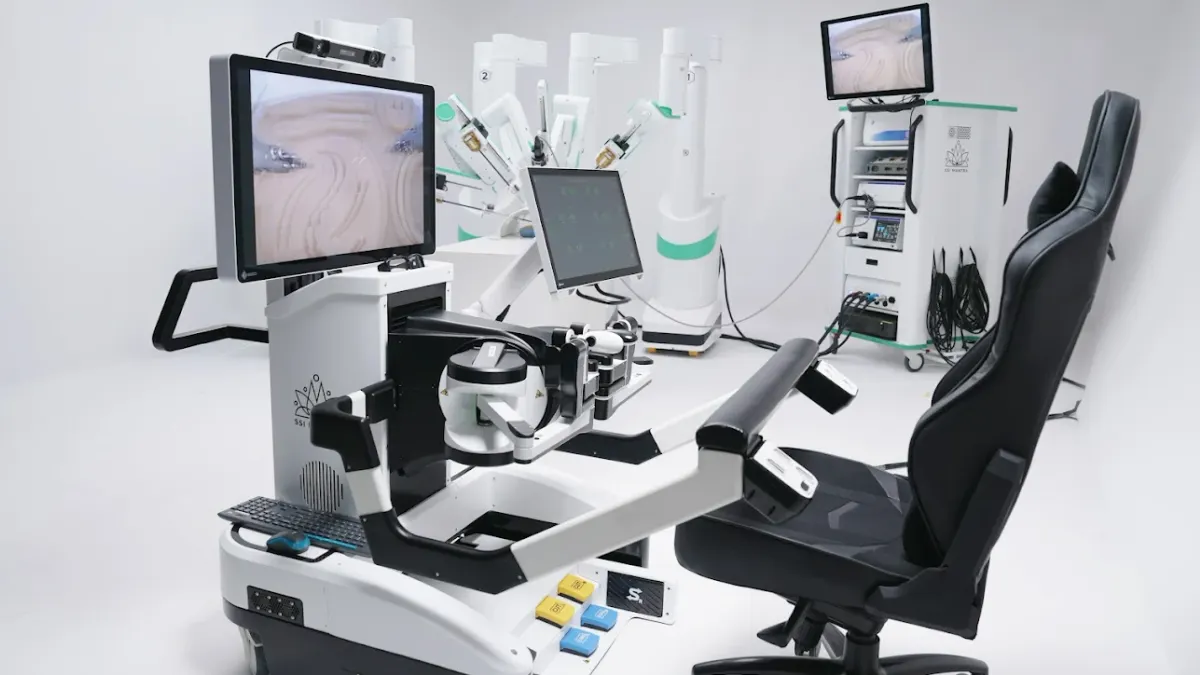
SS Innovations changes course on FDA filing for soft tissue robot
A 510(k) submission now planned for this quarter sets up a potentially faster clearance than the de novo pathway originally planned, the company said.
By Susan Kelly • Oct. 2, 2025 -
FDA seeks feedback on monitoring real-world performance of AI devices
The agency is looking for ways to detect, assess and mitigate changes to the performance of AI-enabled devices over time.
By Nick Paul Taylor • Oct. 1, 2025