FDA: Page 24
-
Abbott recall of heart catheter categorized as Class I event
Abbott plans to win approval for a modified steerable product and bring it to market as a replacement for the recalled device.
By Nick Paul Taylor • July 27, 2023 -
FDA adopts new sterilization standard to support switch from ethylene oxide
The regulator said it added the alternative sterilization method to its database in response to pressure to reduce EtO use and to support supply chain resiliency.
By Nick Paul Taylor • July 26, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA improves 510(k) turnaround times, but PMA waits hit record high: analysis
The time it took for the FDA to reach 510(k) decisions decreased by 24 days, but the agency is still working through a pandemic-driven backlog of premarket approval applications.
By Nick Paul Taylor • July 24, 2023 -
BD gains FDA clearance to relaunch Alaris infusion pump after recalls
The company will fix or replace older devices still in use at hospitals as it ramps up to resume sales in its market-leading infusion business.
By Susan Kelly • July 24, 2023 -
FDA provides safety update on hernia repair mesh as medtech companies face lawsuits
The FDA’s analysis of adverse event reports describes problems including pain, injury and disability.
By Nick Paul Taylor • July 17, 2023 -
AdvaMed says CMS radiotherapy rate cut proposal will limit patient access
The proposed rule includes a 2% reduction in reimbursement, continuing a trend that has seen the CMS cut payments by more than 20% over the past decade, according to the the American Society for Radiation Oncology.
By Nick Paul Taylor • July 17, 2023 -
What the new CMS payment proposals mean for device makers
Ophthalmic device company Glaukos could benefit from the proposed changes, analysts wrote.
By Elise Reuter • July 14, 2023 -
Problem with Draeger transport ventilators categorized as Class I recall by FDA
Draeger contacted customers last month after receiving reports that devices stopped providing ventilation because of depleted batteries.
By Nick Paul Taylor • July 14, 2023 -
Urotronic wins FDA approval to challenge Boston Scientific, Teleflex for prostate market
The company’s Optilume device combines balloon dilation and the delivery of paclitaxel to treat symptoms linked to an enlarged prostate.
By Nick Paul Taylor • July 13, 2023 -
Illumina hit by EU with maximum €432M fine for Grail acquisition
The European Commission called Illumina’s closing of the deal without its approval an “unprecedented” move that undermines its system for regulating the competitive landscape.
By Susan Kelly • July 12, 2023 -
Analysis shows paclitaxel-coated devices unlikely to increase death risk, leading FDA to withdraw warning
The FDA’s decision to remove mortality warnings from device labels is a boost to manufacturers of paclitaxel-coated devices.
By Nick Paul Taylor • July 12, 2023 -
J&J Megadyne unit’s electrode pad recall labeled Class I over burn risk
The problem, which can cause third-degree burns, has been linked to 63 injuries and no deaths.
By Nick Paul Taylor • July 12, 2023 -
Analysts expect a reimbursement boost for Shockwave coronary artery-clearing device
The analysts think growth expectations for the company are too low and have set their earnings estimate for next year 15% above Wall Street’s consensus.
By Nick Paul Taylor • July 11, 2023 -
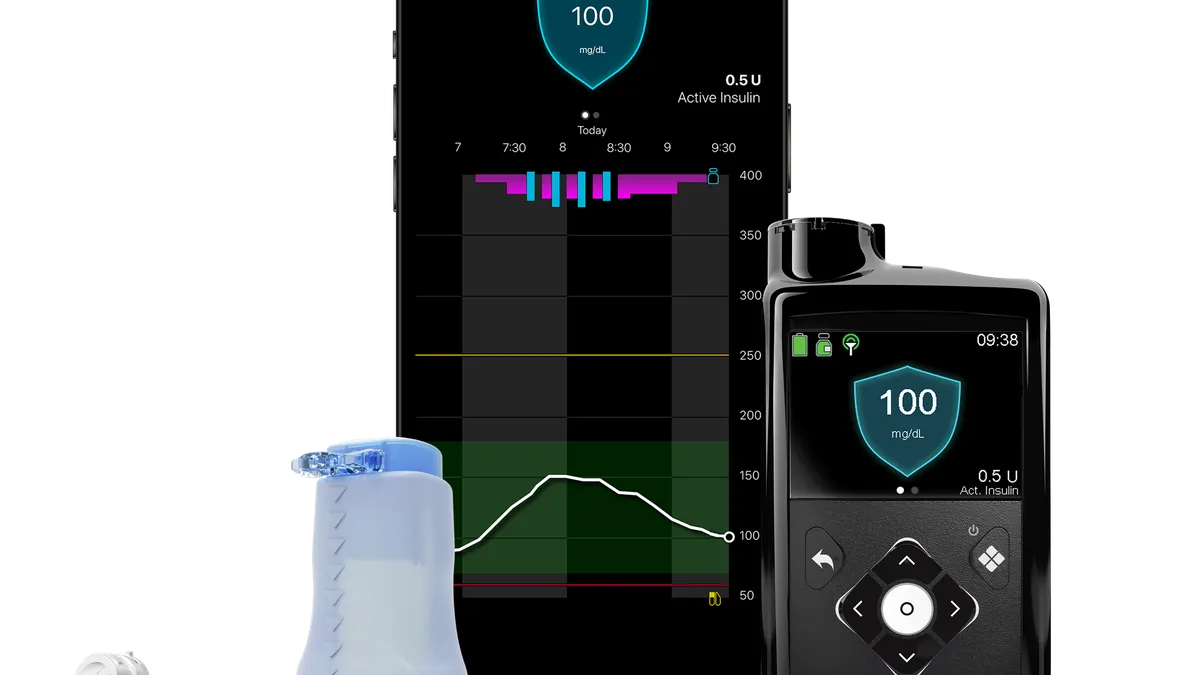
Medtronic secures Medicare coverage for MiniMed 780G insulin pump
Medtronic has begun processing orders and will start shipping devices over the next few weeks.
By Nick Paul Taylor • July 10, 2023 -
European guidelines offer support for renal denervation in hypertension, boosting Medtronic, ReCor
Medtronic hailed the updated guidelines as a “turning point” for the use of renal denervation in treating high blood pressure.
By Nick Paul Taylor • July 6, 2023 -
Startup’s fast-acting wound treatment gel gets FDA nod for use in humans
Cresilon raised $25 million last year as part of a push to expand its wound-gel product for animals into human health.
By Nick Paul Taylor • July 5, 2023 -
EU MDR costs could cause device shortages for children, medical groups warn
The cost of certification under new medical device regulations in Europe is a barrier to manufacturers that make devices for small groups of patients, medical associations said.
By Elise Reuter • June 29, 2023 -

 Retrieved from Istock.
Retrieved from Istock.
FDA seeks feedback on technologies that can enable healthcare at home
The FDA is asking the medtech industry for input on how the agency can support development of devices for use in non-clinical care settings and by diverse patient populations.
By Nick Paul Taylor • June 29, 2023 -
FDA repeats restrictions on NuVasive limb-lengthening device after clearing pediatric use
The agency in March cleared NuVasive to market its Precice limb-lengthening system for use in the femur and tibia of children aged 12 years and up.
By Nick Paul Taylor • June 29, 2023 -

 Carol Highsmith. (2005). "The Apex Building" [Photo]. Retrieved from Wikimedia Commons.
Carol Highsmith. (2005). "The Apex Building" [Photo]. Retrieved from Wikimedia Commons.
FTC proposes updates to merger review that could slow healthcare dealmaking
A new rule proposed by antitrust regulators would ask companies to provide additional information about planned mergers to help the Federal Trade Commission keep pace with increased deal volume and complexity.
By Emily Olsen • June 28, 2023 -
Teleflex recall of separating vascular catheters labeled Class I event
The company began the recall in May after receiving 83 complaints, including 18 reports of injuries, related to the fault.
By Nick Paul Taylor • June 28, 2023 -
Proposed EtO limits garner criticism from device industry, praise from occupational health groups
Lobbyists representing medical device companies say the proposed policy would reduce capacity at commercial sterilizers by as much as 50%.
By Elise Reuter • June 27, 2023 -
CMS proposes pathway to earlier breakthrough device coverage
The voluntary plan would allow manufacturers to address evidence gaps through studies designed to answer specific questions.
By Susan Kelly • Updated June 23, 2023 -

 Retrieved from Owlet on June 22, 2023
Retrieved from Owlet on June 22, 2023
Owlet receives FDA clearance for prescription pulse oximetry baby sock
Clearance of the new system settles a controversy that began last year when Owl sold a similar pulse oximetry device over the counter, but was ordered to pull it from sale and seek regulatory approval as a medical device.
By Nick Paul Taylor • June 22, 2023 -
Opinion
Proposed breast cancer screening recommendations don’t go far enough
A proposal by a government-sponsored task force calling for a 24-month spacing between mammograms fails to address the latest research and may further exacerbate disparities and mortality outcomes, argues Henry Izawa, head of FujiFilm Healthcare Americas.
By Henry Izawa • June 21, 2023