FDA: Page 29
-
Medtech trends in 2023
Medtech regulation outlook in 2023: Faster approvals among priorities as AI moves to fore
Leaders in the medtech field weigh in on the prospects and challenges for product regulation in 2023.
By Peter Green • Updated Jan. 26, 2023 -
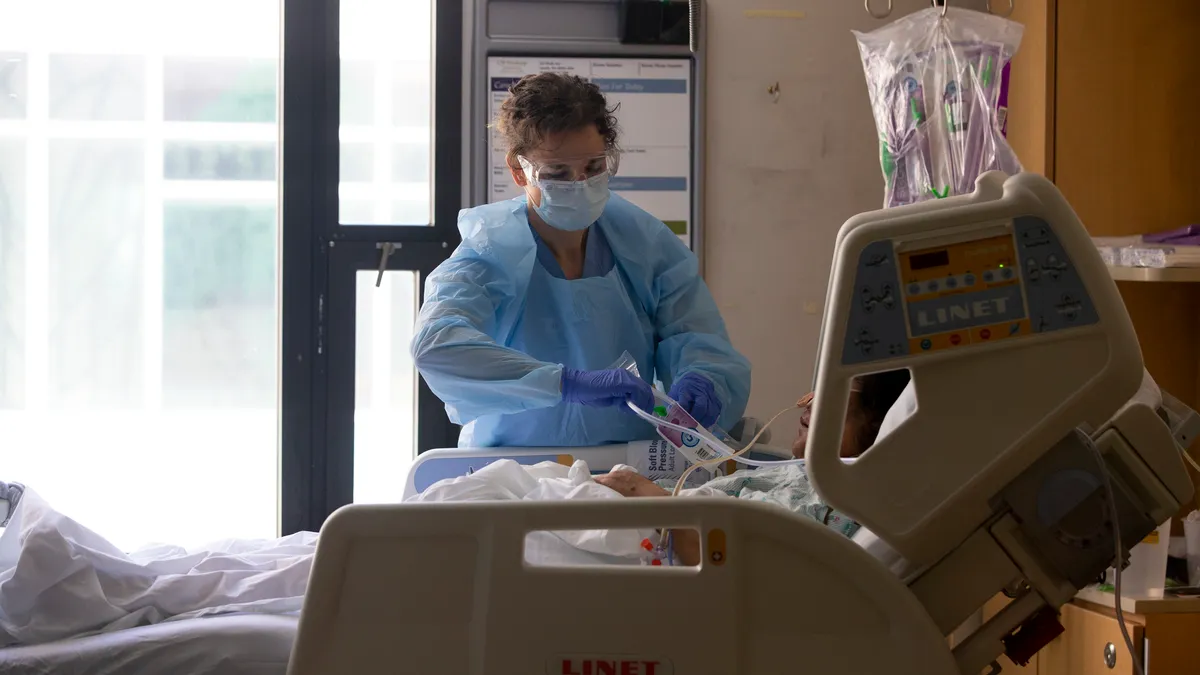
Getinge heart pump gets third Class I recall after patient death
Customers were alerted to problems with Cardiosave devices late last year after Getinge received complaints about an issue linked to four serious injuries and one death.
By Nick Paul Taylor • Jan. 26, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Insulet reports personal-data leak of 29,000 insulin pump customers
In a recall communication to users, Insulet shared information with its website performance and marketing partners through cookies and other trackers embedded in its website.
By Nick Paul Taylor • Jan. 24, 2023 -
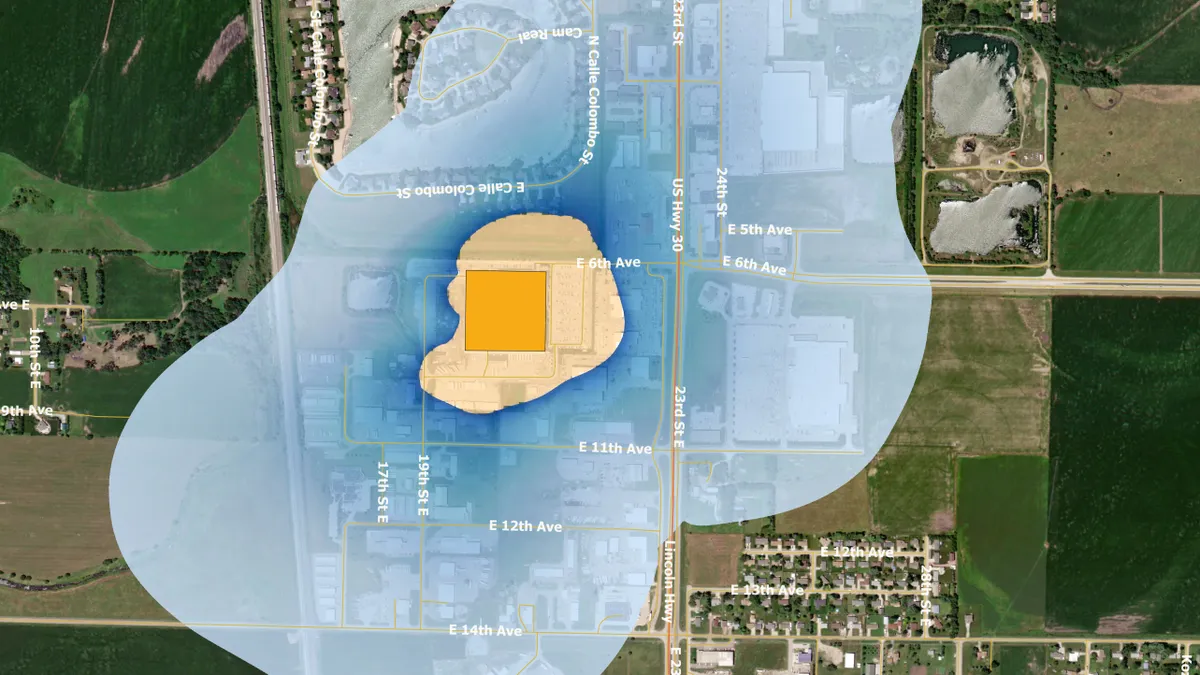
 EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
Ethylene oxide regulation may disrupt device supply, AdvaMed warns White House
The trade group has asked President Joe Biden to consider the potential threat to patient care if facilities are shut down.
By Nick Paul Taylor • Jan. 23, 2023 -
FDA demands additional data on Abbott rival to Medtronic balloon, setting back approval timeline
Abbott’s partner Surmodics is “evaluating options” to reduce its spending while preparing a response to the regulator’s requests.
By Nick Paul Taylor • Jan. 20, 2023 -
Medtech hazards: Problems with Philips’ recall put safety of home-use devices at top of danger list
Patient safety group ECRI is challenging manufacturers to provide easy-to-follow device registration instructions and write simply worded recall notices.
By Nick Paul Taylor • Jan. 19, 2023 -
Abbott’s FDA approval for TAVI device tees up challenge to Edwards, Medtronic: analysts
Abbott has captured about 9% of the European market and aims to become a “credible third player” in the U.S.
By Nick Paul Taylor • Jan. 18, 2023 -
Rethinking 510(k): Studies show risk of using recalled devices as predicates for FDA clearance
Two studies found devices cleared through the FDA’s 510(k) pathway that listed recalled predicate devices were more likely to face recalls themselves.
By Nick Paul Taylor • Jan. 11, 2023 -
European Commission formalizes plan to extend MDR transition out to 2027, 2028
Having accepted that notified body capacity “remains insufficient,” the Commission wants to give manufacturers more time to get their devices designated under MDR.
By Nick Paul Taylor • Jan. 9, 2023 -
Vivos bounces back from FDA rejection to land 510(k) clearance for sleep apnea treatment
Shares in Vivos increased by more than 150% on the day of the news.
By Nick Paul Taylor • Jan. 6, 2023 -
Roche receives first European IVDR certificate for companion diagnostic
Receipt of the certificate clears Roche to sell a companion diagnostic for immune checkpoint inhibitors in the European Union.
By Nick Paul Taylor • Jan. 5, 2023 -
Diagnostic testing reform missing from Congress’ year-end spending bill
A provision that would have brought in-vitro diagnostic tests and lab-developed tests under one regulatory framework was not included in the omnibus spending bill.
By Elise Reuter • Dec. 21, 2022 -
MDR costs are driving manufacturers to pull products from European market: report
The report adds to evidence that makers of products used for pediatric patients and rare diseases have been particularly hard hit.
By Nick Paul Taylor • Dec. 20, 2022 -
Fraud risk increased among Medicare DME suppliers who enrolled under pandemic waivers: GAO
Of 208 providers that had their Medicare enrollment revoked between 2020 and 2022, 83% were durable medical equipment suppliers, according to a report from the Government Accountability Office.
By Elise Reuter • Dec. 19, 2022 -
Click wins FDA breakthrough designation for prescription digital migraine therapy
The designation would give Click Therapeutics expedited review of its preventive treatment for episodic migraine in adults.
By Nick Paul Taylor • Dec. 19, 2022 -
Healthcare groups urge Congress to pass diagnostic testing reform before year’s end
After the VALID Act was dropped from an FDA funding package, healthcare groups are asking legislators to add it to a year-end funding bill.
By Elise Reuter • Dec. 13, 2022 -
EU Health Commissioner proposes MDR delay to prevent medical device shortages
Plans to delay MDR certification to 2027 for high-risk devices and 2028 for others may still not be enough to stave off shortages.
By Nick Paul Taylor • Updated Dec. 23, 2022 -
Cochlear’s $120M purchase of hearing-implant maker stirs European scrutiny
The European Commission said the merger “threatens to significantly affect competition in the market for cochlear implants and bone conduction solutions.”
By Nick Paul Taylor • Dec. 9, 2022 -
AdvaMed asks FDA to withdraw divisive draft guidance on LASIK surgery
Some comments were more supportive, with the American Optometric Association calling the agency’s proposal “timely and beneficial.”
By Nick Paul Taylor • Dec. 8, 2022 -
FDA outlines benefits and risks of emerging augmented and virtual reality medical devices
AR/VR may increase access to healthcare and make procedures less invasive, but could potentially cause cybersickness and head and neck strain.
By Nick Paul Taylor • Dec. 8, 2022 -
18M projected to lose Medicaid coverage at end of COVID-19 emergency
Many people who are currently enrolled in Medicaid will transition to other coverage, but 3.8 million people will completely lose insurance, according to the Robert Wood Johnson Foundation.
By Susan Kelly • Dec. 6, 2022 -
Getinge heart support devices join FDA shortage list as raw-materials supply slows production
Maintenance cycles for pumps and batteries have been extended and Getinge asked hospitals to share underutilized devices.
By Nick Paul Taylor • Dec. 5, 2022 -
Vulnerability in BD’s infusion pump flagged to US cybersecurity agency
An attacker with physical access to the device could exploit the vulnerability to change the configuration settings or disable the pump.
By Nick Paul Taylor • Updated Dec. 6, 2022 -
The 10 biggest medtech stories of 2022
MedTech Dive reporting this year has explored how companies maintained momentum even amid supply shortages and rising inflation rates.
By MedTech Dive staff • Dec. 3, 2022 -
European biomedical group backs delaying MDR certification deadline amid ‘looming crisis’
Pushing back the recertification deadline may buy time to address problems such as the shortage of capacity at certifying organizations.
By Nick Paul Taylor • Dec. 1, 2022