FDA: Page 3
-
Biolinq gets FDA de novo nod for intradermal glucose sensor
The device, called Biolinq Shine, is indicated for people with Type 2 diabetes who don’t depend on insulin.
By Elise Reuter • Updated Sept. 29, 2025 -
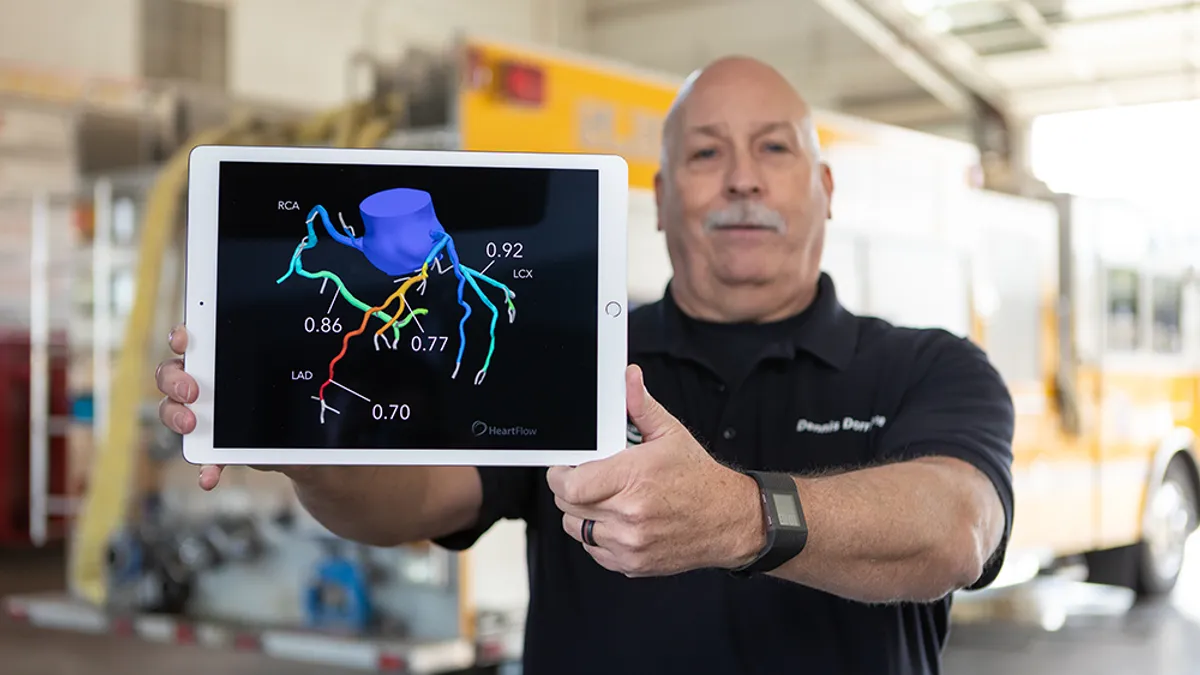
Heartflow receives FDA clearance for updated plaque analysis platform
The updated algorithm shows a 21% improvement in plaque detection, compared to the original version.
By Nick Paul Taylor • Sept. 25, 2025 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
J&J’s Abiomed starts third heart pump controller recall since June
As of Aug. 27, the company had reported five serious injuries and no deaths associated with the issue.
By Nick Paul Taylor • Sept. 24, 2025 -
Medtronic wins FDA approval for urinary incontinence device
Analysts said Altaviva is an attractive option compared to rival products, informing their belief that Medtronic can capitalize on a large market opportunity.
By Nick Paul Taylor • Sept. 22, 2025 -
FDA posts early alert about Abbott’s Tactiflex Ablation Catheter
Catheter tips came off three damaged devices during surgery but none of the patients suffered serious injuries or died.
By Nick Paul Taylor • Updated Sept. 22, 2025 -
FDA warns on unauthorized blood pressure, infant monitoring devices
Many of these devices currently sold over-the-counter do not have marketing authorization, the agency said in a notice.
By Elise Reuter • Updated Sept. 18, 2025 -
BD expands Class I recall to cover 15 more Alaris pump infusion sets
BD, which has not received any complaints related to the issue, previously discontinued the devices affected by the expanded recall.
By Nick Paul Taylor • Sept. 17, 2025 -
Patients call for more medical device user fee funding, FDA staffing
Commenters to the FDA also advocated for safety reforms and for industry funds to be used for post-market monitoring.
By Elise Reuter • Sept. 11, 2025 -
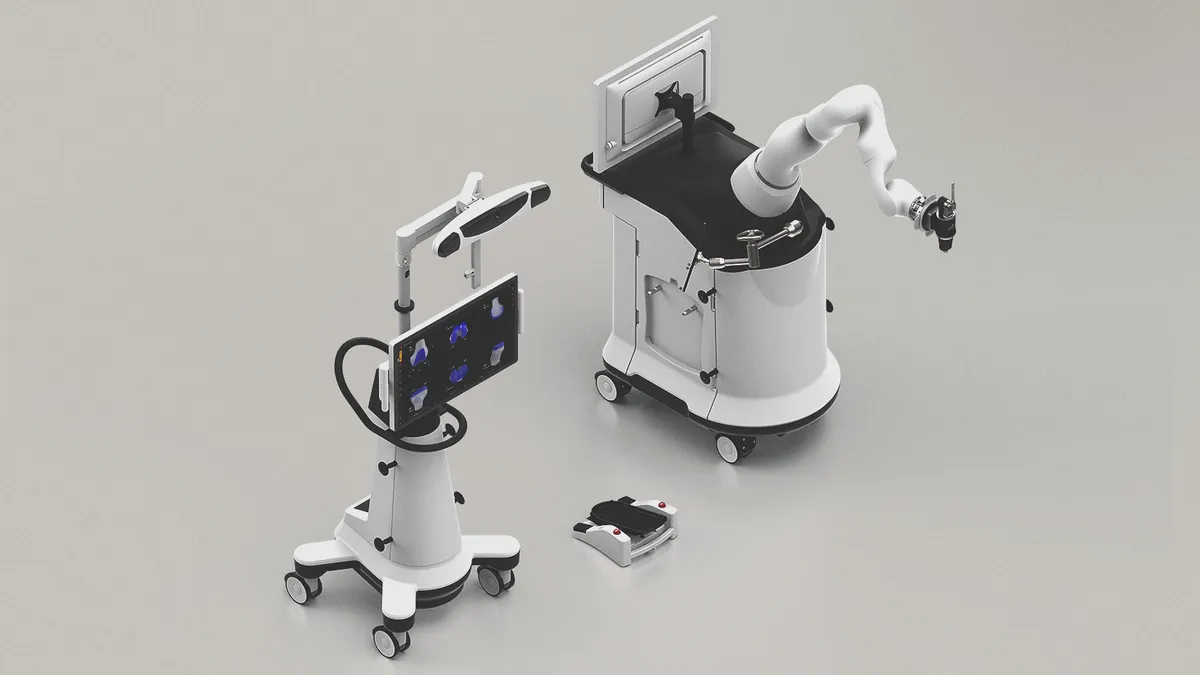
Microbot receives 510(k) clearance for endovascular robot
The company will now complete final commercial activities in preparation for targeting the 2.5 million peripheral vascular procedures performed in the U.S. each year.
By Nick Paul Taylor • Sept. 9, 2025 -
At Senate hearing, lawmakers express dissatisfaction with RFK Jr.’s vaccine moves
Robert F. Kennedy Jr. faced criticism Thursday even from Republican lawmakers over changes to federal vaccine policy as well as a leadership crisis at the CDC.
By Delilah Alvarado , Jonathan Gardner • Sept. 4, 2025 -
Over 1,000 HHS employees call for RFK Jr. to step down
The letter cites turmoil at the CDC, which is reeling from the firing of Director Susan Monarez and the departure of at least four high-level leaders last week.
By Rebecca Pifer Parduhn • Sept. 4, 2025 -
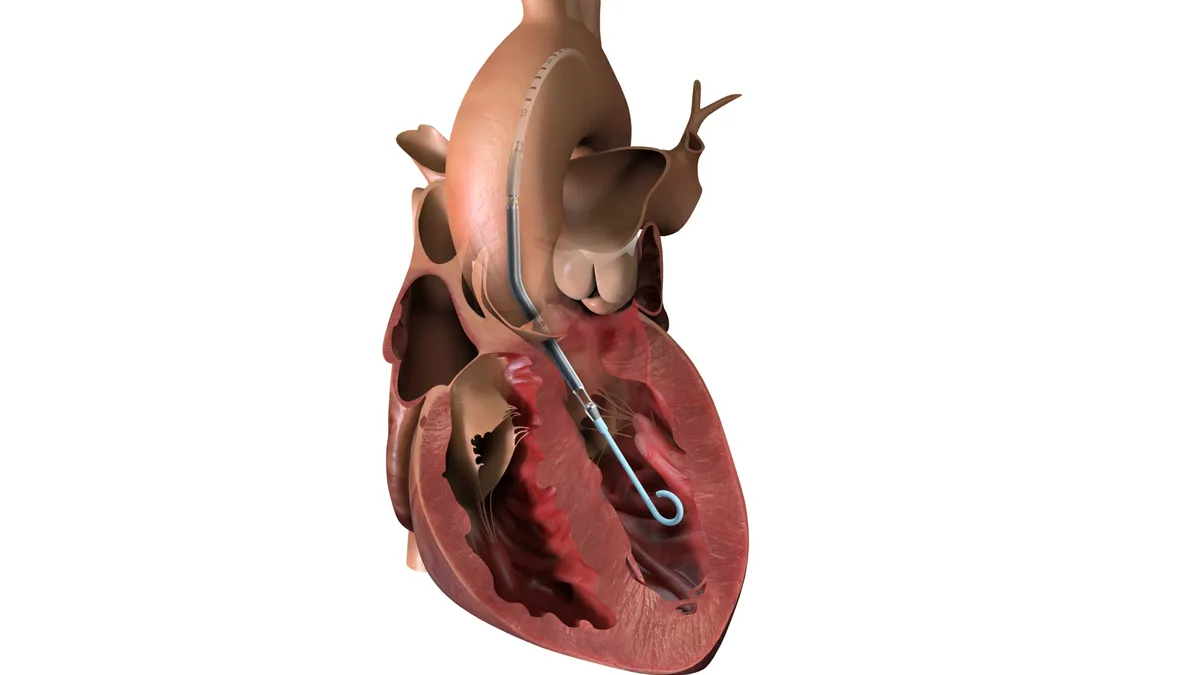
Kardium receives FDA OK for pulsed field ablation system
The company raised $250 million in July to expand its manufacturing capabilities and establish multiple teams to support the launch.
By Nick Paul Taylor • Sept. 4, 2025 -
Medtronic advances Abbott partnership with FDA nod
The company received FDA authorization to pair its 780G insulin pump with a glucose sensor made by Abbott, and to use the device for Type 2 diabetes.
By Elise Reuter • Sept. 2, 2025 -
J&J removes some Abiomed heart pump controllers from market
Abiomed reported one death associated with a pump driver circuit assembly that does not meet current specifications. The recall affects 69 controllers, a J&J spokesperson said.
By Susan Kelly • Aug. 28, 2025 -
Deep Dive
4 medtech topics to watch for the rest of 2025
From M&A to MDUFA and the developing market in renal denervation, MedTech Dive covers the key issues to watch in the final months of the year.
By Ricky Zipp , Susan Kelly , Elise Reuter • Aug. 25, 2025 -
Boston Scientific recalls carotid stents over manufacturing defect
The FDA said the defect could injure blood vessels, damage the stent or release debris that could travel to the brain and cause a stroke.
By Nick Paul Taylor • Aug. 25, 2025 -
More than 1,100 devices have received the FDA’s breakthrough designation
The FDA updated its list of breakthrough devices as medtech groups lobby for faster Medicare coverage of products with the designation.
By Elise Reuter • Aug. 21, 2025 -
Q&A
How Aktiia built the first over-the-counter cuffless blood pressure monitor
Aktiia received FDA clearance in July for the first cuffless blood pressure monitor authorized to be sold over the counter.
By Elise Reuter • Aug. 20, 2025 -
NeuroOne gains FDA clearance for trigeminal nerve ablation
The minimally invasive system uses radiofrequency energy to target nerve tissue causing facial pain.
By Susan Kelly • Aug. 20, 2025 -
Medtronic recall of heart vent catheters tied to 3 serious injuries
The FDA said the request to quarantine the devices followed reports of the products “resisting shape retention when being bent.”
By Nick Paul Taylor • Aug. 19, 2025 -
Boston Scientific updates instructions of devices linked to 17 deaths
The update covers devices used in procedures to implant the company’s Watchman heart device.
By Nick Paul Taylor • Aug. 8, 2025 -
Boston Scientific tells users about defibrillator problem linked to deaths, injuries
A company spokesperson said in a statement to MedTech Dive that direct causation between the deaths and calcification of the leads cannot be assumed or confirmed.
By Nick Paul Taylor • Aug. 7, 2025 -
Medical device industry says future MDUFA hikes unsustainable
Device lobbyists’ priorities contrasted with patient advocates, who sought an increase in user fees and more funds for postmarket safety.
By Elise Reuter • Aug. 5, 2025 -
News roundup
Monogram robot marks autonomy milestone; Stereotaxis catheter cleared
Monogram Technologies conducted its first fully autonomous robotic knee replacement surgery, while Stereotaxis won FDA clearance for a robotically navigated electrophysiology mapping catheter.
By Susan Kelly • July 31, 2025 -
Cardiosense wins FDA clearance for wearable heart monitor
The device captures various types of heart data that could be used in AI models for cardiovascular parameters with the aim of lowering barriers to monitoring.
By Nick Paul Taylor • July 31, 2025