FDA: Page 34
-
Boston Scientific says it’s investigating allegations of anti-bribery law violations
The company says the issue is centered on its operations in Vietnam.
By Nick Paul Taylor • Aug. 10, 2022 -
Eargo’s Q2 revenue slides amid decline in hearing-aid shipments
The company’s gross system shipments fell by more than 8,000 when compared to the year-earlier period.
By Ricky Zipp • Aug. 9, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA’s hearing-aid guidance clears regulatory review amid push by lawmakers for OTC sales
The move comes after the agency proposed the creation of a new category of over-the-counter hearing aids in October.
By Nick Paul Taylor • Aug. 9, 2022 -
Senate passes $740B bill to lower healthcare costs after months of wrangling
While Republicans successfully challenged a planned $35 insulin price ceiling for patients on private insurance, the law still has implications for makers of diabetes devices.
By Nick Paul Taylor • Aug. 8, 2022 -
FDA’s breakthrough device designations poised for another record year
At the midpoint of the year, the FDA had granted 129 designations, suggesting it will break the previous full-year record of 206.
By Nick Paul Taylor • Aug. 8, 2022 -
SEC accuses surgical implant business of pulling forward sales to hit targets
According to the SEC, Surgalign, known at the time as RTI Surgical, shipped future orders ahead of schedule to pull forward revenues and mask disappointing sales figures.
By Nick Paul Taylor • Aug. 5, 2022 -
Biden administration declares monkeypox a public health emergency
Federal health officials said Thursday that U.S. monkeypox testing capacity is at about 80,000 tests per week, although only 10% of capacity is currently being used.
By Ricky Zipp • Aug. 4, 2022 -
Hospitals have low level of accountability for connected device breaches
Of the 43% of organizations that reported a data breach in the past two years, 88% said at least one connected device was a contributing factor to the breach, according to a new report.
By Rebecca Pifer Parduhn • Aug. 4, 2022 -
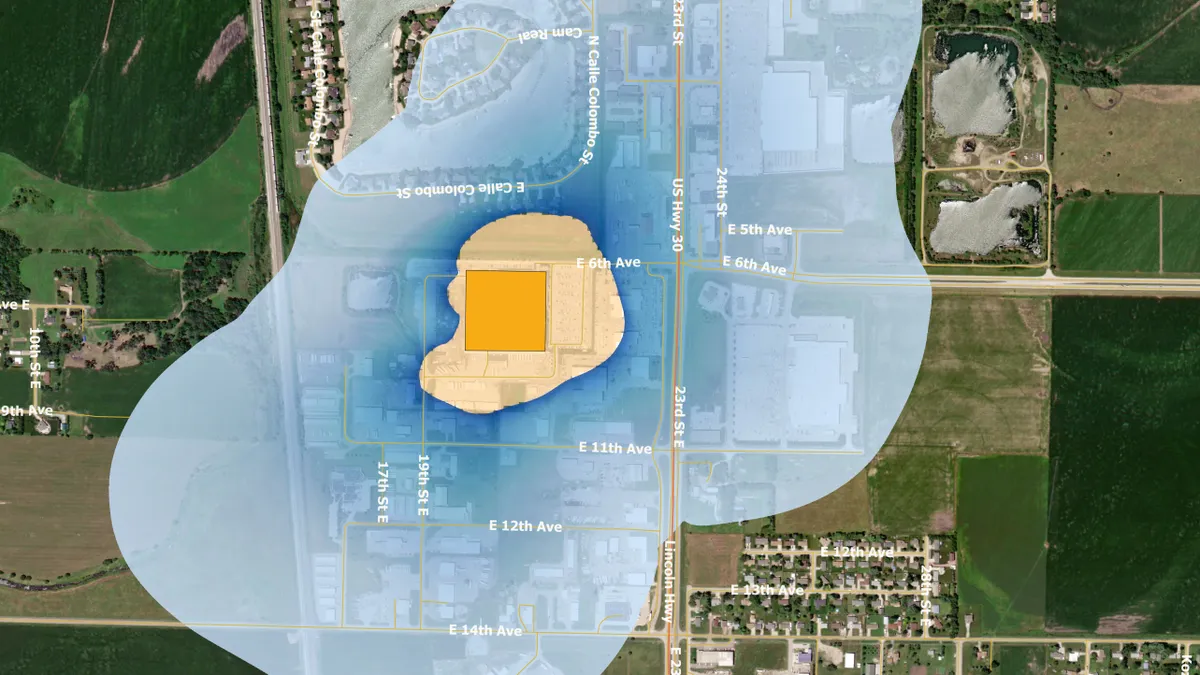
 EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
EPA. (2022). "https://www.epa.gov/hazardous-air-pollutants-ethylene-oxide/forms/columbus-nebraska-becton-dickinson-pharmaceutical" [Photo]. Retrieved from EPA.gov.
EPA names BD, Edwards and Medtronic on list of higher-risk sterilization facilities
AdvaMed said ethylene oxide is vital for prevention of serious infections and shutting facilities could disrupt the supply of medical devices.
By Nick Paul Taylor • Updated Aug. 5, 2022 -
6 questions for the medtech industry if Congress doesn’t reauthorize FDA user fees on time
If Congress doesn’t pass the legislation before Sept. 30, furloughs and delays lie ahead for the agency.
By Elise Reuter • Aug. 3, 2022 -
 Retrieved from Siemsens Website on July 01, 2022
Retrieved from Siemsens Website on July 01, 2022
Siemens Healthineers stumbles as lockdowns, falling COVID-19 sales and rising costs hit business
Lockdowns in China caused a steep decline in equipment orders and a big setback to the firm’s core diagnostic business, even as the company expects to meet full year revenue targets.
By Nick Paul Taylor • Aug. 3, 2022 -
DOJ sues Idaho over near-total abortion ban in first post-Roe challenge
In a suit filed in Idaho’s southern district, the Department of Justice claimed that Idaho’s “near-absolute” abortion ban blocks clinicians from providing necessary care in medical emergencies as required by federal law.
By Sydney Halleman • Aug. 2, 2022 -
North American Diagnostics latest to recall COVID-19 tests, gets Class I label from FDA
The recall adds to a list of medtech firms that have been forced to withdraw illegally distributed COVID-19 tests, amid concerns they could yield false results.
By Ricky Zipp • Aug. 2, 2022 -
Illumina vows to fight EU efforts to block $8B Grail merger
Illumina acquired the liquid biopsy company in 2021, despite pending anti-competitiveness investigations on both sides of the Atlantic.
By Elise Reuter • Updated Aug. 2, 2022 -
Abortion clinics go mobile, seeking flexibility amid patchwork of state restrictions
Mobile clinics may open the healthcare system to patients barred by state laws from accessing abortion services.
By Rebecca Pifer Parduhn • Aug. 1, 2022 -
FDA extends Class I UDI submission deadline, exempts consumer health devices from requirements
The deadline extension should help manufacturers submit device identification data to an agency database, while freeing over-the-counter consumer devices from needless regulation.
By Nick Paul Taylor • Aug. 1, 2022 -

 Retrieved from Medridien Bioscience website on August 01, 2022
Retrieved from Medridien Bioscience website on August 01, 2022
FDA reauthorizes Meridian’s COVID-19 test after changes to enable omicron detection
The renewed emergency use authorization comes as SD Biosensor works to close its $1.53 billion takeover of Meridian.
By Nick Paul Taylor • Aug. 1, 2022 -
Q&A
Friday Q&A: Eargo CEO Christian Gormsen talks OTC rule, challenging market leaders, DOJ settlement
New FDA regulations expected soon would boost firms like Eargo, allowing millions of Americans without hearing aids to buy them for less and without a prescription.
By Ricky Zipp • July 29, 2022 -
LASIK again under microscope as FDA seeks comment on improving patient communication
Amid concerns patients are not receiving information on the risks and benefits of laser eye surgery, FDA’s draft guidance aims to protect consumers.
By Nick Paul Taylor • July 28, 2022 -
Medtech industry applauds as Senate passes $280B measure to bolster U.S. chip production
Industry leaders say the funding will strengthen U.S. innovation and speed the deployment of life-changing devices to patients.
By Elise Reuter • Updated July 28, 2022 -
MedTech Europe pushes for semiconductor prioritization as US moves to increase local supply
The trade group wants political action to stop patient harm from “a growing divergence” between semiconductor supply and demand.
By Nick Paul Taylor • July 27, 2022 -
Biotronik to pay $12.95M to settle kickback allegations of 'lavish' meals, abuse of training programs
Federal prosecutors accused Biotronik of abusing a new employee program by paying physicians to provide “excessive” training and of funding lavish meals.
By Nick Paul Taylor • July 26, 2022 -
Philips and U.S. Justice Dept. in consent decree talks on sleep apnea, ventilator recall
The company made the announcement Monday during a second-quarter earnings call but provided few details.
By Ricky Zipp , Elise Reuter • Updated July 25, 2022 -
FDA to expand use of remote regulatory assessments beyond the pandemic
The agency plans to keep using remote assessments, saying they’ve been a “valuable tool” during the pandemic.
By Elise Reuter • July 25, 2022 -
iRhythm, Alphabet unit Verily get FDA clearances for Zio Watch and software system
Bolstered by an AI algorithm, the companies’ partnership is a step closer to catching up with the Apple Watch.
By Ricky Zipp • July 25, 2022