FDA: Page 35
-
Test makers urge House, Senate to include diagnostic reforms in user-fee bill
AdvaMedDx, Abbott and Roche are among companies asking lawmakers to address the “urgent public health issue.”
By Nick Paul Taylor • July 21, 2022 -
Smiths Medical's recall of syringe infusion pumps labeled Class I event after injuries, death
The company is asking customers to return devices if abnormal circuit board behavior causes the pump to stop.
By Nick Paul Taylor • July 21, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
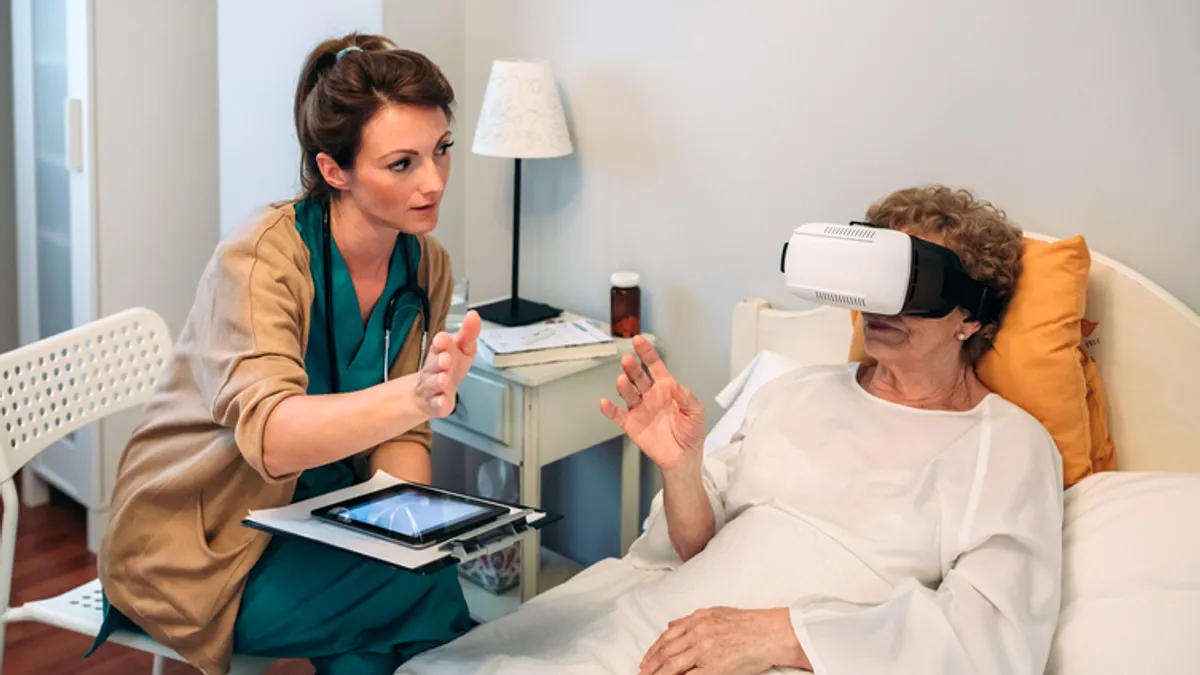
FDA patient advisers grapple with consent, oversight of virtual-reality devices in healthcare
The advisory group addressed patient consent in surgeries using augmented reality tools and how to distinguish clinical uses of virtual reality from entertainment products.
By Elise Reuter • July 20, 2022 -
Defibrillators, chest drains added to FDA's list of device shortages
The defibrillator supply disruption reflects both increased demand and problems sourcing a component, part or accessory.
By Nick Paul Taylor • July 20, 2022 -
Notified bodies have yet to issue MDR certificates for 85% of legacy devices: survey
The EU's Medical Device Regulation certification process is taking twice as long as the old directive pathway and smaller companies are struggling to get started, the poll shows.
By Nick Paul Taylor • July 18, 2022 -
FDA faces furloughs if Senate misses August deadline for user fee bill
If legislation isn’t passed before the August recess, the FDA would have to furlough workers, slowing its review of medical device and pharmaceutical products.
By Elise Reuter • July 15, 2022 -
Medtronic warns European healthcare providers of safety problem in defibrillators
The warning adds to Medtronic’s growing list of product safety issues over the last 18 months.
By Ricky Zipp • July 15, 2022 -
Q&A
Friday Q&A: Ian Lipkin, Columbia epidemiologist, talks COVID-19 testing as U.S. sees another wave
Lipkin explained why he’s not a fan of antigen tests, and stressed the need to keep PCR testing a core part of the pandemic response.
By Elise Reuter • July 15, 2022 -
Lack of payment pathway clarity hinders digital therapeutics, analysts say
The report warns of a sector beset by payment issues, “snake oil” and “middling adoption.”
By Nick Paul Taylor • July 15, 2022 -
EC publishes IVDR guidance in absence of completed Eudamed database
The commission recently announced a timeline for complete implementation of Eudamed, aiming for the second quarter of 2024.
By Ricky Zipp • July 13, 2022 -
Quest launches monkeypox PCR test as U.S. attempts to catch up with rising cases
The company estimates it will be able to process 30,000 tests per week by the end of July. As of July 12, there were 929 confirmed cases in the U.S., the CDC reported.
By Ricky Zipp • July 13, 2022 -
Abbott gets FDA breakthrough tag for deep brain stimulation system in depression
The breakthrough device designation builds on a series of clinical trials of other systems that have yielded mixed results.
By Nick Paul Taylor • July 13, 2022 -
CMS makes 'market-moving proposals' to some medtech Medicare rates, say analysts
BTIG analysts warned in a recent report that the changes “might curtail usage of some higher-dollar” medical devices.
By Nick Paul Taylor • July 12, 2022 -
European Commission targets spring of 2024 for fully functional Eudamed database
The Commission is planning a two-phase transition to mandatory use of the database after it goes live.
By Nick Paul Taylor • July 11, 2022 -
Medtronic recalls over 1M dialysis catheters due to malfunction
The recall is the latest in a growing list of product safety issues for Medtronic, including Class I recalls and an FDA warning letter for its diabetes unit.
By Ricky Zipp • July 11, 2022 -
Ex-Mazor executive charged by SEC with insider trading over Medtronic's $1.6B buyout
Doron Tavlin, who was Mazor’s vice president of business development, is accused by the agency of profiting from his inside knowledge of the pending takeover.
By Nick Paul Taylor • July 11, 2022 -
CMS proposes national rates for cardiac monitoring; iRhythm's stock jumps
The proposed rule could end a years-long saga for the cardiac market, although pricing specifics may change in the final version.
By Ricky Zipp • July 8, 2022 -
High-risk IVD tests rules finalized by European Commission despite industry calls for change
MedTech Europe pushed back against some of the requirements, but the Commission has retained disputed parts of the document.
By Nick Paul Taylor • July 8, 2022 -
Getinge recalls anesthesia machines due to cracked, broken suction power switches
The FDA labeled the recall a Class I event, marking Getinge’s fifth Class I recall since September.
By Ricky Zipp • July 7, 2022 -
Senators press FDA to finalize OTC hearing aids, accuse industry of 'astroturf' campaigns
The two cross-aisle politicians said they want to “expand access, reduce costs, and ensure a robust new market for safe and effective OTC hearing aids” and accused the device manufacturing lobby of “harming American consumers.”
By Elise Reuter • July 6, 2022 -
American Contract Systems' COVID-19 test recall gets Class I label from FDA
Off-site assembly by workers who may not have been properly trained prompted the company to recall COVID-19 tests amid concerns they may yield false results.
By Nick Paul Taylor • Updated July 6, 2022 -
AliveCor ECG patent ruling sets stage for block on Apple Watch imports
Apple Watch imports to the U.S. could be barred if ruling by International Trade Court judge is finalized; Judge says Apple Watch infringes two cardiogram patents.
By Nick Paul Taylor • July 5, 2022 -

 Retrieved from LiveMetric website on July 01, 2022
Retrieved from LiveMetric website on July 01, 2022
LiveMetric's blood-pressure 'smartwatch' device gets FDA clearance
The wristwatch-like device will be made available through health systems, insurers and self-insured employers to ease continuous monitoring of blood pressure and avoid white-coat syndrome.
By Nick Paul Taylor • July 1, 2022 -
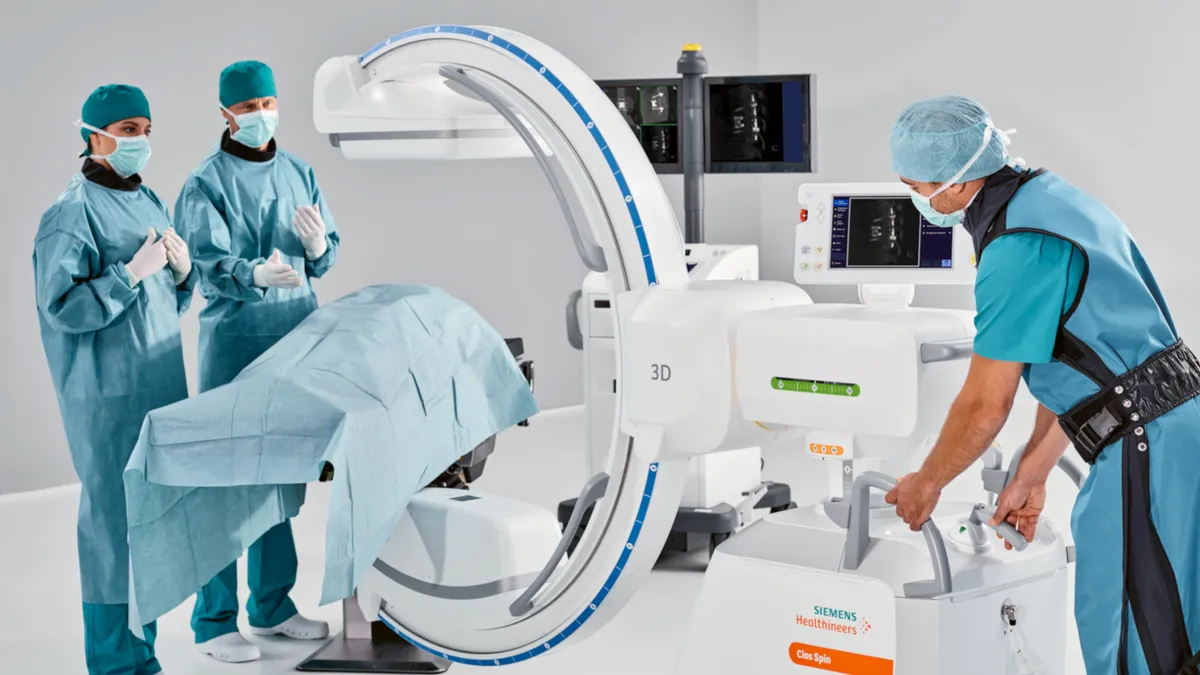
 Retrieved from Siemsens Website on July 01, 2022
Retrieved from Siemsens Website on July 01, 2022
Intuitive secures FDA clearance for lung biopsy robot featuring Siemens' imaging tech
The FDA clearance is an “incremental positive” for Intuitive, wrote analysts at RBC Capital Markets, adding that they see Ion as “an important leg of growth for the company.”
By Nick Paul Taylor • July 1, 2022 -
As Philips works to replace or repair recalled devices, supply problems are slowing it down
Logistical challenges mean patients won’t know when they will get a replacement sleep apnea or ventilator device until “at best a few weeks before it is delivered,” Philips executives said.
By Ricky Zipp • June 30, 2022