FDA: Page 36
-
Commercial labs to ramp up monkeypox testing in 'coming weeks'
The Food and Drug Administration said in a Wednesday meeting that public health labs currently have a throughput of 10,000 tests per week, and the addition of five reference labs will expand that capacity to 60,000 tests per week.
By Elise Reuter • June 29, 2022 -
Sleep apnea devices show 'very low' amount of degraded foam, Philips says
Philips found evidence of foam breaking down in about 2% of 60,847 returned first generation DreamStation devices. The company said the problem was more prevalent in devices that used an ozone cleaning method.
By Ricky Zipp • June 28, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
BD recalls emergency vascular access devices over risk of delayed care
Because patients who need intraosseous access are often critically ill, Becton Dickinson warned delays to care caused by the device problems could lead to death.
By Nick Paul Taylor • June 27, 2022 -
UnitedHealth's Optum looks to cut down on unnecessary lab testing
Optum says roughly 13 billion clinical lab tests are performed each year and 30% are unnecessary.
By Samantha Liss • June 27, 2022 -
Supreme Court overturns Roe v. Wade, ending constitutional right to abortion
26 states are certain or likely to end abortion rights, making abortion effectively illegal in half of the country, according to the Guttmacher Institute.
By Sydney Halleman • June 24, 2022 -
Baxter ventilator recall labeled Class I event by FDA after 2 deaths
Baxter initiated the recall after learning that an improper setup could reduce oxygen flow to in-home users, causing serious injury or death.
By Nick Paul Taylor • June 24, 2022 -
Patient death prompts another recall of Medtronic's HVAD System
The company initiated another recall for the troubled heart pump after a patient died when two batteries simultaneously stopped working, the company said, adding to a list of safety problems with the device.
By Ricky Zipp • June 23, 2022 -
FDA opens consultation on tissue removal devices to reduce cancer risk
The agency is seeking feedback on draft guidance to help manufacturers of tissue containment systems reduce risk that cancerous tissue could leak during procedures.
By Nick Paul Taylor • June 22, 2022 -
Deep Dive
As cross-state telemedicine waivers expire, virtual care advocates focus on long-term policy changes
There’s a complication: No one solution to the U.S.’ patchy physician licensing infrastructure has universal buy-in.
By Rebecca Pifer Parduhn • June 22, 2022 -
Senseonics lands CE mark for 6-month CGM implant, teeing up Q3 launch in Europe
Senseonics is looking to the longer-lasting implant to re-energize its fight in a market dominated by Abbott Laboratories and Dexcom.
By Nick Paul Taylor • June 17, 2022 -
User fee package goes to Senate with lab-developed test, OTC hearing aid provisions
The Senate HELP committee passed its version of the FDA user fee bill by a 13-9 vote. It includes an overhaul of diagnostic testing regulations and a requirement to create a category of over-the-counter hearing aids.
By Elise Reuter • June 15, 2022 -
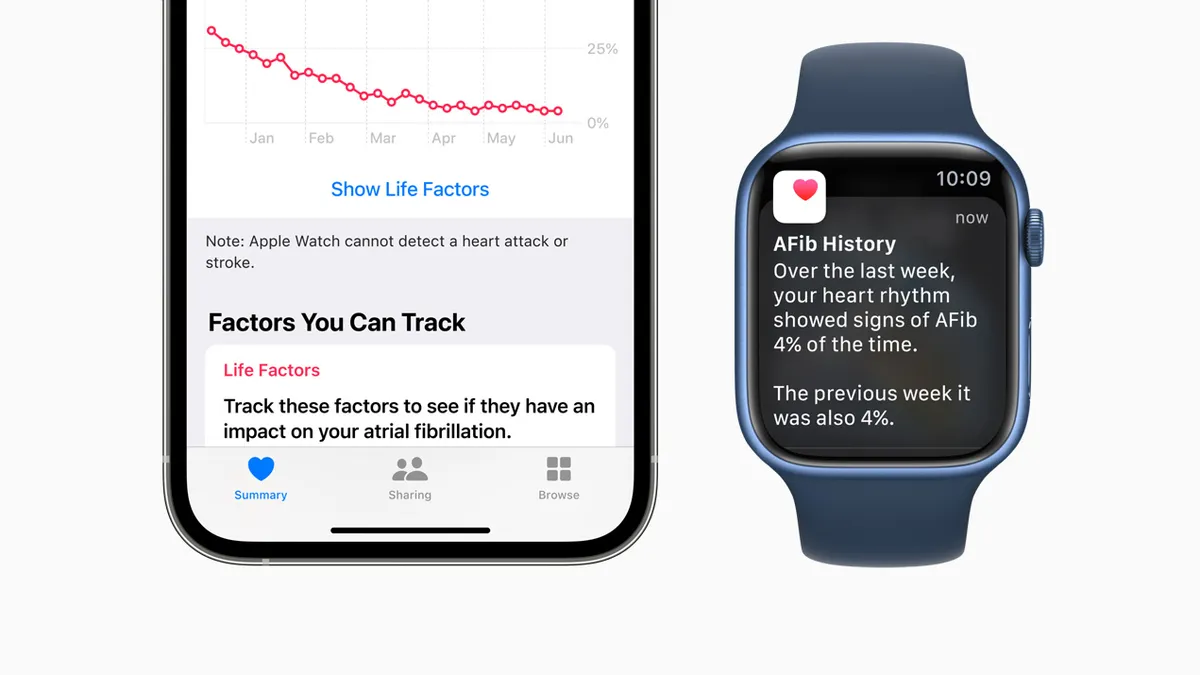
Apple Watch monitoring features for AFib, Parkinson's cleared by FDA
With the new feature, atrial fibrillation patients may have an easier way to track the frequency of the condition over time and see whether lifestyle changes may have positive effects.
By Nick Paul Taylor • June 14, 2022 -
Dräger's breathing system filter recall gets Class I label from FDA after ventilation obstruction
An obstruction in one of the company’s SafeStar 55 devices resulted in a patient being resuscitated after becoming hypoxic.
By Nick Paul Taylor • June 13, 2022 -
House passes FDA user-fee package, bolstering cybersecurity, clinical trial diversity for medical devices
By a 392-28 vote, the House of Representatives passed its version of the legislation, which would renew the Food and Drug Administration's ability to collect user fees for the next five years.
By Elise Reuter • June 9, 2022 -
Medtronic: No new patient injury, death reports since April letters warning of HVAD defect
The company’s HeartWare Ventricular Assist Device System received a Class I label from the FDA following two patient injuries and one death.
By Ricky Zipp • Updated June 14, 2022 -
GE Healthcare ventilator battery recall tied to over 1,500 complaints
Ventilators risk shutting down prematurely — cutting the flow of oxygen to patients — as backup batteries may run out of power earlier than expected.
By Nick Paul Taylor • Updated June 28, 2022 -
FDA mulls pilot program on alternative sterilization for medical devices
The proposed initiative is a response to global supply chain constraints and is intended to support sterilization supply chain resiliency, the agency said.
By Nick Paul Taylor • June 8, 2022 -
Q&A
Dexcom CEO Sayer on G7 FDA submission, developing a 15-day sensor, M&A plans
Kevin Sayer spoke about the company's back-and-forth with the FDA on the G7 CGM system, possibly expanding from a 10-day to a 15-day sensor and on a report that Dexcom was in talks to buy Insulet.
By Ricky Zipp • June 7, 2022 -
DHS warns cybersecurity vulnerabilities in Illumina software could affect test results
Three of the flaws outlined by the Department of Homeland Security received the highest risk score. Vulnerabilities could allow attackers to remotely alter the results generated by Illumina products.
By Nick Paul Taylor • June 6, 2022 -
Getinge's recall of 69,000 stents labeled as Class I event by FDA amid complaints, injuries
The recall relates to the separation of the balloon or catheter hub during the removal of the delivery system from the patient.
By Nick Paul Taylor • June 3, 2022 -
FDA to start accepting all pre-submissions for in vitro diagnostics
The agency had previously declined pre-submission requests unless they were related to COVID-19. Test developers should expect an extended timeline for reviews amid a backlog of pandemic-related submissions.
By Elise Reuter • June 1, 2022 -
Medtronic expects diabetes unit's struggles to continue as rivals grow sales, launch products
The company forecasts revenues for the diabetes business to decrease 8% to 10% in the first quarter of its fiscal year and 6% to 7% for the full year.
By Ricky Zipp • June 1, 2022 -
BD's Pyxis medication dispenser gets fifth DHS cybersecurity alert in 5 years
The company said there are no known public exploits that specifically target a password vulnerability and that it's working to address the problem.
By Nick Paul Taylor • June 1, 2022 -
Recalled devices surged in first quarter, driven by BD connectors
A recall of 288 million Becton Dickinson connectors for catheter ports drove up the total number of recalled devices in the quarter. Mislabeling was the top reason for FDA alerts over the three-month period, a Sedgwick report found.
By Nick Paul Taylor • June 1, 2022 -
Abbott's Libre 3 glucose monitor gets FDA clearance as CGM market intensifies
The 14-day glucose sensor is smaller and thinner than its predecessor, and will give Abbott a head start on rival Dexcom’s newest continuous glucose monitor.
By Nick Paul Taylor • May 31, 2022