FDA: Page 59
-
At FDA's request, Penumbra recalls catheter device after 14 patient deaths
"There's no way around the fact that this is a disappointing blow to the company's credibility given the positive commentary we've received from the company since the initial July warning notice," J.P. Morgan analysts wrote.
By Ricky Zipp • Dec. 17, 2020 -
Stryker, Zimmer shift strategies to capitalize on growing ASC market
Now that Medicare has caught up to private payers opening up coverage policies, experts see procedure migration away from hospitals accelerating. Pricing pressure is a risk, though.
By Ricky Zipp • Dec. 16, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Breakthrough designations go to renal denervation devices in latest FDA batch
ReCor and SoniVie disclosed the regulatory privileges within a day of each other, boosting their efforts to enter a market targeted by Medtronic.
By Nick Paul Taylor • Dec. 16, 2020 -
FDA lays out alternative 510(k) path criteria for 3 device types
The agency finalized guidance documents covering several product categories — spinal plating systems, orthopaedic bone screws and magnetic resonance coils — as it pushes forward with its Safety and Performance Based Pathway.
By Susan Kelly • Dec. 15, 2020 -
FDA grants EUA for first fully at-home COVID-19 test without a prescription
The rapid antigen test from Australian company Ellume will be available over the counter for people with or without symptoms and delivers results in about 20 minutes.
By Susan Kelly • Dec. 15, 2020 -
Deep Dive
MDR-IVDR bottleneck persists as EU launches 1st Eudamed module
"Very few notified bodies at this moment in time are taking new products on under the old directive, because a medical device doesn't just get certified overnight," an official at Ireland's National Standards Authority said.
By Nicholas Wallace • Dec. 14, 2020 -

 Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Master Sgt. Hecht, Matt. (2020). [Photograph]. Retrieved from Flickr.
Trump admin defends national stockpile as PPE, equipment shortages persist
"We’re not the Walmart that you can walk into on a daily basis to get your standard needs to treat patients and get PPE. We are a 911 response team," an HHS official said, adding the stockpile hasn't denied a single state request.
By Rebecca Pifer Parduhn • Dec. 14, 2020 -
Roche and Siemens COVID-19 antibody tests shine in FDA accuracy roundup
Abbott's tests seemed to perform slightly worse than the best serology assays but comparably to kits from some other leading diagnostic players such as Beckman Coulter.
By Nick Paul Taylor • Dec. 14, 2020 -
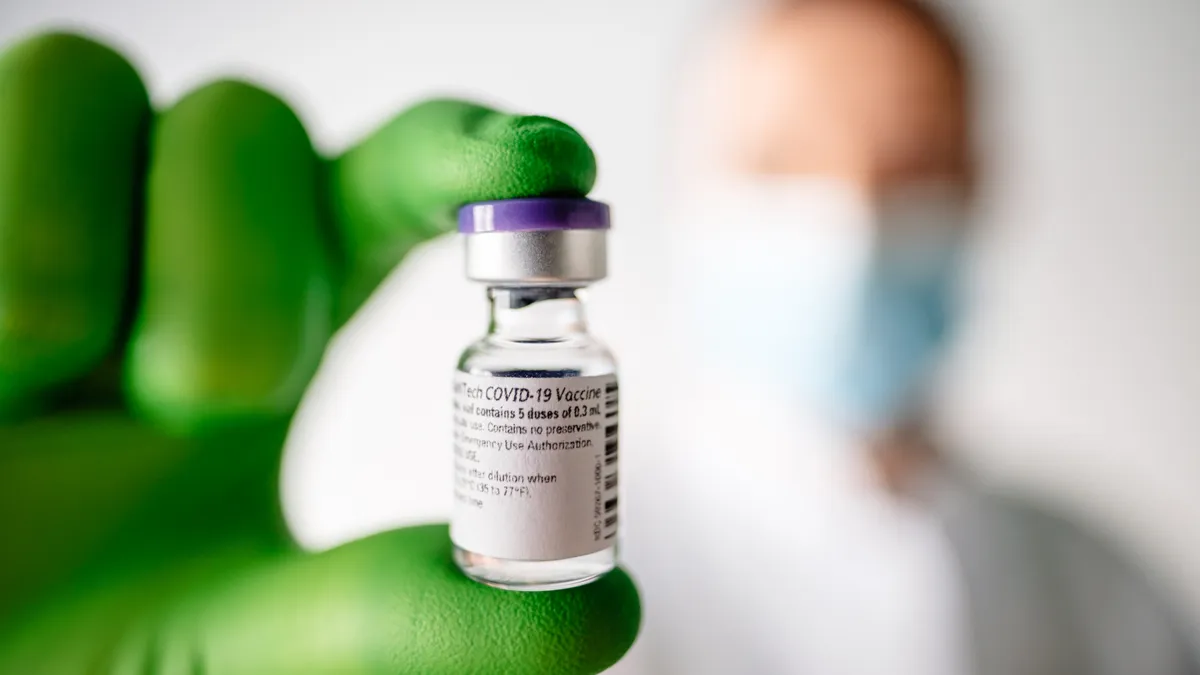
In milestone, FDA clears coronavirus vaccine from Pfizer, BioNTech for emergency use
Vaccinations are expected to begin within days of the historic decision, which follows early approvals in the U.K., Canada and Mexico.
By Jonathan Gardner • Dec. 11, 2020 -
Trump admin proposes easing HIPAA to foster value-based care, COVID-19 contact tracing
One goal in revamping the privacy regulations was to make information sharing more efficient, especially during health crises like the opioid epidemic and the coronavirus outbreak, according to top health officials.
By Rebecca Pifer Parduhn • Dec. 11, 2020 -
UK shares post-Brexit device guidance as uncertainty around EU split looms
MHRA this week outlined which medtech manufacturers will need to register and when. The timeline is determined by the previously disclosed grace period which ends Jan. 1, 2021.
By Nick Paul Taylor • Dec. 11, 2020 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

FDA advisers back Pfizer, BioNTech vaccine, clearing way for emergency approval
A panel of independent experts agreed the benefits of vaccination outweigh the risk, convinced by strong data showing the companies' shot to be 95% effective in preventing COVID-19.
By Ned Pagliarulo • Updated Dec. 10, 2020 -
LabCorp gets EUA for first at-home collection COVID-19 test with no prescription
The lab giant is selling the kit through its consumer-focused Pixel website and may expand into retail channels. The agency is hoping the over-the-counter diagnostic will spur more testing.
By Nick Paul Taylor • Dec. 10, 2020 -
Roche, Thermo Fisher ally with COVID-19 vaccine developers on antibody testing
The companies are working with Moderna and the University of Oxford to quantitatively measure the antibody levels of recipients of the shots.
By Nick Paul Taylor • Dec. 10, 2020 -
Dive Awards
Regulatory Disruption of the Year: HHS lab developed test policy
The surprise move to no longer require FDA premarket review for laboratory developed tests spurred backlash across a spectrum of public health experts and industry.
By Greg Slabodkin • Dec. 9, 2020 -
Dive Awards
The MedTech Dive Awards for 2020
From the relentless demand for COVID-19 tests to the pressure on medtechs' traditional business lines, the industry has faced unprecedented market forces this year.
By MedTech Dive Team • Dec. 9, 2020 -
Hospitals prepare to reveal prices online come Jan. 1
The rule requires hospitals to prepare a list of pricing information for 300 "shoppable services," such as total knee replacements. However, experts question if the data will benefit consumers.
By Samantha Liss • Dec. 8, 2020 -
ACA defender Becerra tapped for Biden's HHS chief
The California attorney general has voiced opposition to provider consolidation in his state, alleging anticompetitive practices by regional powerhouse Sutter Health and denying a hospital system merger.
By Shannon Muchmore • Dec. 7, 2020 -
Endologix AAA graft to go before FDA panel over potential life-threatening leak risk
The agency also released postmarketing data reinforcing an increased likelihood of blood leaking into the aneurysm for patients with the company's AFX endovascular grafts.
By Susan Kelly • Dec. 7, 2020 -

 Retrieved from Quest Diagnostics, PRNewswire on May 29, 2020
Retrieved from Quest Diagnostics, PRNewswire on May 29, 2020
Quest gets first FDA nod for at-home collection coronavirus-flu combo test
The lab giant is using Roche’s diagnostic and instrument, which received an emergency use authorization in September, with its own process for testing samples collected by consumers.
By Nick Paul Taylor • Dec. 7, 2020 -
Siemens COVID-19 test gets high marks on FDA sensitivity list, beating Abbott, BD and Roche
A PerkinElmer test still holds the top position among coronavirus diagnostics least likely to return false negatives.
By Nick Paul Taylor • Dec. 4, 2020 -
CDRH headed for 'reset' in 2021 after COVID-19 derailed priorities, Shuren says
After a "massive increase" in work due to the pandemic, the device center anticipates continuing to manage coronavirus projects while also focusing on MDUFA V and new programs.
By Ricky Zipp • Dec. 3, 2020 -
CMS adds hip replacements, other surgeries to ASC list in final rule
A representative from Stryker applauded the changes and said the rule will encourage providers to consider the shift to ambulatory surgery centers.
By Ricky Zipp • Updated Dec. 4, 2020 -
EU underscores remote notified body audit policy amid pandemic
MedTech Europe has pressed the Commission to expand such reviews to the incoming MDR and IVDR regs, but a new Q&A notes the scope is limited to the directives and devices considered "clinically necessary" during the crisis.
By Nick Paul Taylor • Dec. 3, 2020 -
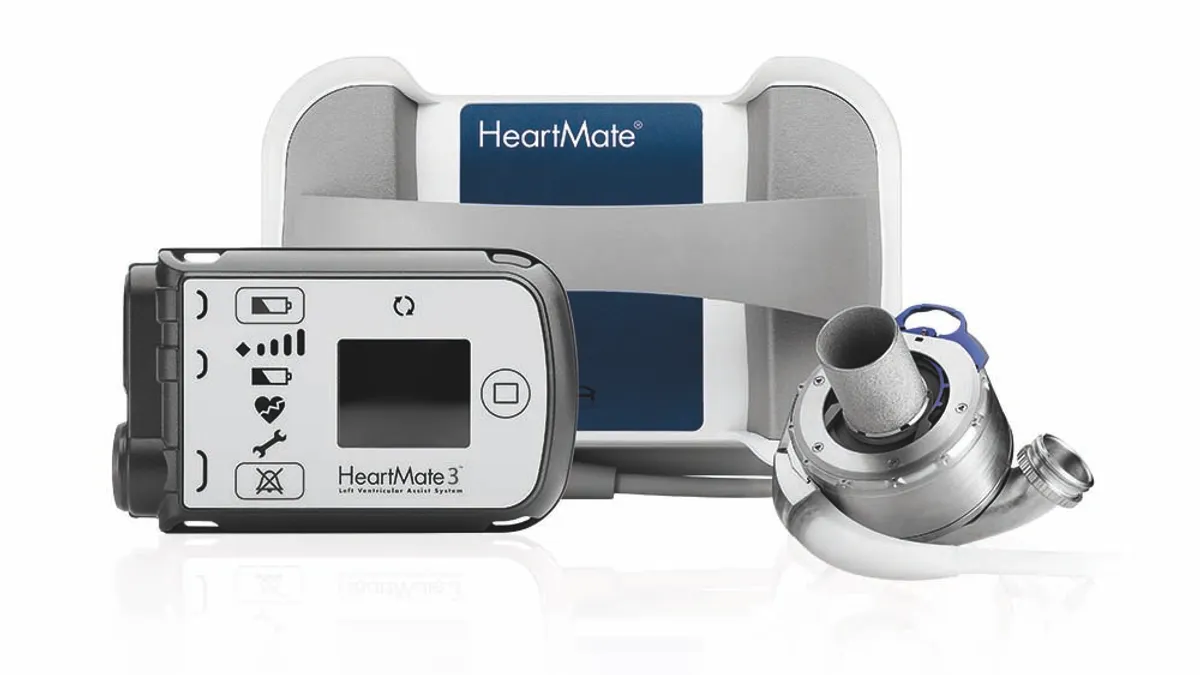
CMS finalizes expanded artificial heart, ventricular assist device coverage
Medicare will cover artificial hearts outside of clinical studies and align VAD coverage criteria with medical practice. Cowen called the decision good for Abbott and Medtronic.
By Nick Paul Taylor • Dec. 2, 2020