FDA: Page 93
-
Texas judge rules ACA unconstitutional
The end of the Affordable Care Act would kill the medical device tax, but the ruling will almost certainly be appealed to a higher court.
By Tony Abraham • Dec. 14, 2018 -
FDA: Benefits beat risks with unregulated medical software
The agency reviewed safety and best practices for technologies like e-prescribing tools and mobile wellness apps, building on prior 21st Century Cures guidances addressing software as a medical device.
By Maria Rachal • Dec. 14, 2018 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA walks back new device class definitions after industry blowback
AdvaMed and the Medical Device Manufacturers Association scored a temporary win after arguing the proposed revisions to the Class III definition could shift more devices into the high-risk category.
By David Lim • Dec. 14, 2018 -
Medtech industry cheers as NAFTA 2.0 edges forward
Trade groups heralded improved transparency in the recently-signed USMCA, which includes a provision that would enforce more timely reimbursement decision-making by national bodies.
By Nick Paul Taylor • Dec. 14, 2018 -
FDA touts progress in move toward global device audits
Participating manufacturing sites have spiked this year with 2,629 cumulatively added as of the third quarter of 2018, up from 777 at the end of 2017.
By David Lim • Dec. 13, 2018 -
FDA adds information on device BLAs to user fee guidance
The revised document features additional information on the fees for device biologics license applications.
By Nick Paul Taylor • Dec. 13, 2018 -
FDA rates GE ventilator safety guard recall as Class I event
The recall was triggered after the tech giant discovered the device has the potential to disconnect from the patient's breathing circuit.
By Nick Paul Taylor • Dec. 13, 2018 -
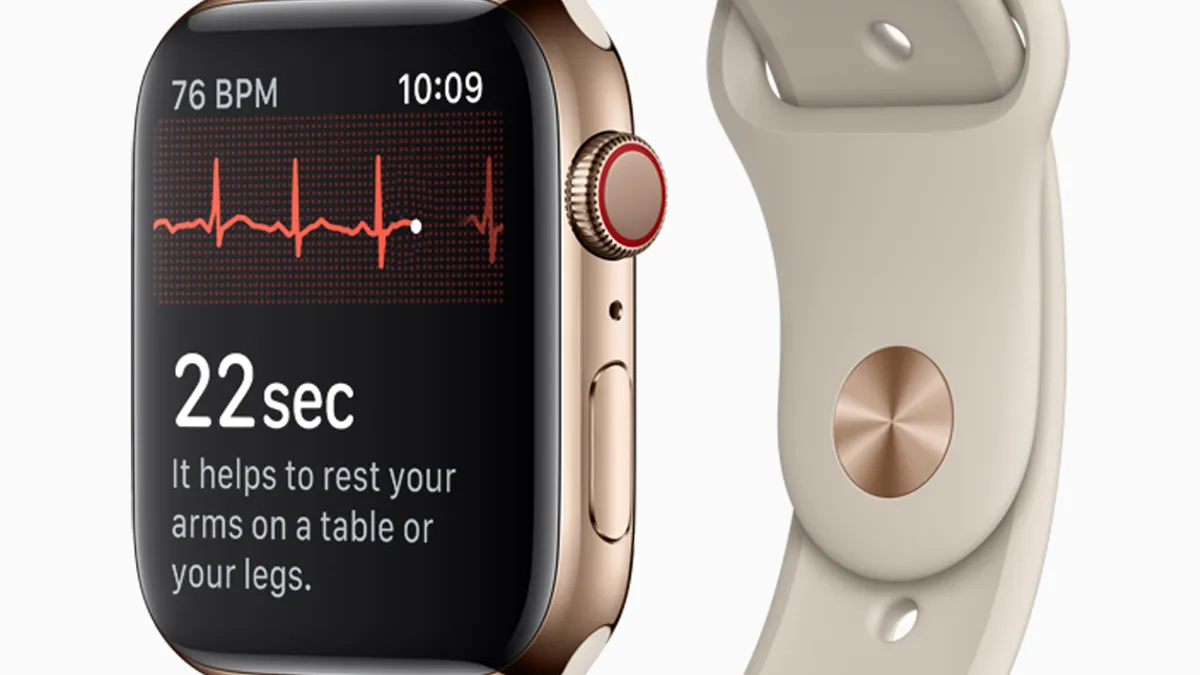
 Retrieved from Apple on September 12, 2018
Retrieved from Apple on September 12, 2018
Apple EKG, artificial iris among FDA 2018 device decisions
De Novos are on the rise, but both premarket approvals and 510(k)s appear to be slightly down from 2017, according to nearly year-end data from FDA.
By Meg Bryant • Dec. 12, 2018 -
Australia plans reclassification of medical software to better reflect risk
The country's regulator thinks the current system underestimates the threat posed by software as a medical device.
By Nick Paul Taylor • Dec. 12, 2018 -
Lawmakers impatient for ONC interoperability rule
"It is hard to explain to people that Congress provided the tools necessary for doctors and patients to better coordinate their care through the sharing of patient data, but nothing has changed," Rep. Michael Burgess, R-Texas, said at a House health subcommittee hearing.
By Rebecca Pifer Parduhn • Dec. 11, 2018 -
Thermo Fisher gets FDA nod for plazomicin immunoassay
The antibiotic, marketed as Zemdri, is used to treat patients with complicated urinary tract infections.
By Susan Kelly • Dec. 11, 2018 -
Duodenoscope studies find higher-than-expected contamination rate, FDA says
Across studies run by Olympus, Fujifilm and Pentax, 6% of sampled devices were contaminated with microorganisms including pathogenic strains such as E. coli.
By Nick Paul Taylor • Dec. 11, 2018 -
Cybersecurity priorities laid out by key House panel
The House Energy and Commerce Committee issued a report outlining six strategies for strengthening the nation's defenses against cybersecurity vulnerabilities with a focus on medical devices in healthcare.
By Susan Kelly • Dec. 10, 2018 -
FDA to revisit transvaginal mesh issues at February meeting
The agency said it may consider additional regulatory actions based on public feedback and Feb. 12 advisory panel discussions.
By Susan Kelly • Dec. 10, 2018 -
FDA guide unveils vision for cancer diagnostics
The proposal came the same day lawmakers released an updated draft bill that would grant FDA authority to oversee in vitro clinical tests, such as test kits and laboratory developed tests.
By David Lim • Dec. 7, 2018 -
AdvaMed, MITA offer feedback on CDRH guidance priorities
Industry groups backed some of the FDA's device center proposals for fiscal year 2019 and added a few of their own.
By Maria Rachal • Dec. 7, 2018 -
FDA seeks to boost use of De Novo pathway with proposed rule
The increased clarity intended for the regulatory framework and application process could incentivize manufacturers to establish new predicates, aligning with the agency's plan to phase out older ones.
By Maria Rachal • Dec. 5, 2018 -
FDA endorses open database for genetic variants
The agency said it hopes the move will encourage database administrators to submit genetic variant information to publicly accessible databases, with the aim of spurring advancements in precision medicine.
By Susan Kelly • Dec. 5, 2018 -
Bipartisan bill introduced in Senate to penalize Medicaid gaming
The incoming leaders of the Senate Finance Committee want to set monetary penalties for companies that misclassify products within Medicaid.
By Andrew Dunn • Dec. 5, 2018 -
ACLA appeals lab payment suit to higher court
A watchdog report on Medicare lab payments will not help the industry's case, analysts said.
By David Lim • Dec. 5, 2018 -
FDA classifies Medtronic's ventilator correction a Class I recall
The software update addresses user interface problems that led Australian regulators to suspend the device.
By Nick Paul Taylor • Dec. 5, 2018 -
OIG finds Medicare overpaid $34M to device suppliers over three years
The overpayment stems from improper billing for prosthetics and other devices used at inpatient facilities.
By Nick Paul Taylor • Dec. 5, 2018 -
Medicare could overpay billions for lab tests: GAO
Medicare's new method of calculating payment rates for laboratory tests was intended to save the government money but could instead end up costing CMS as much as $10.3 billion over three years.
By Susan Kelly • Dec. 3, 2018 -
Dive Awards
Person of the Year: Bakul Patel, FDA
The regulator has drawn praise from Commissioner Scott Gottlieb and others for his Software Pre-Cert Program work.
By David Lim • Dec. 3, 2018 -
Dive Awards
Disruption of the Year: EU regulatory overhaul
It’s a "pendulum swing," said Rajesh Misra, principal in KPMG's technology enablement practice. "More and more as we talk to clients, this is coming across as a bigger transformation than they originally thought … impacting [their] entire life cycle."
By Meg Bryant • Dec. 3, 2018



