Cardiac: Page 7
-
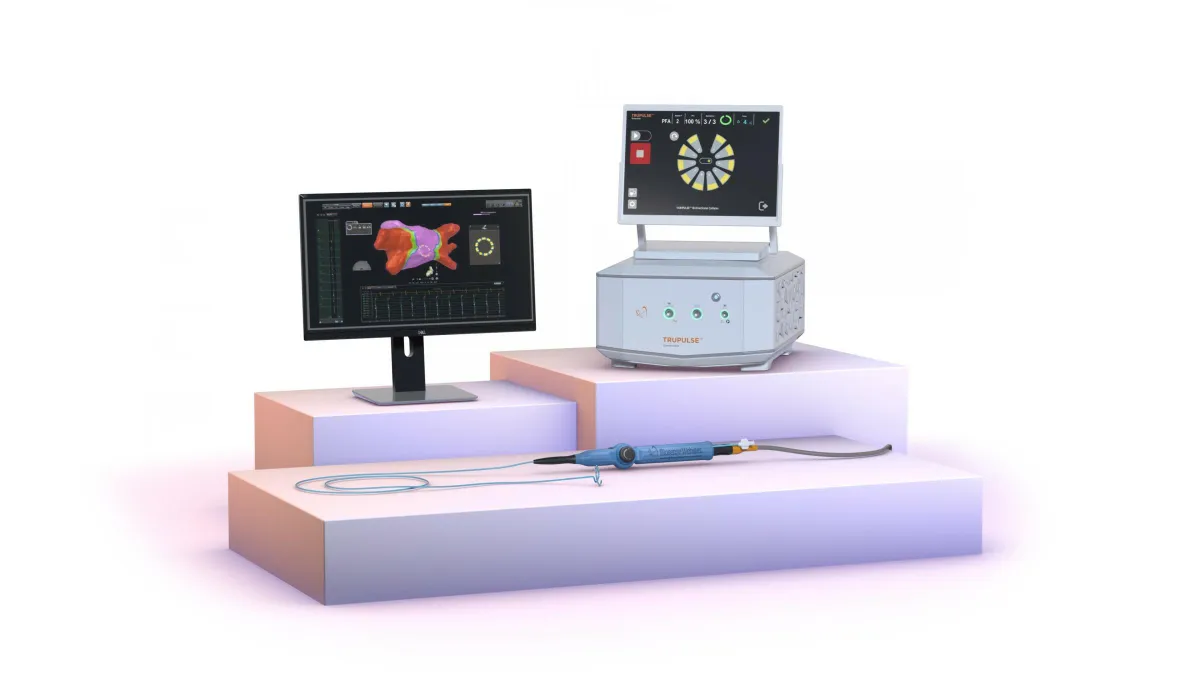
The PFA race is heating up. Here’s where the market stands after J&J’s approval.
Boston Scientific, Medtronic and J&J are moving quickly to secure their place in the fast-growing pulsed field ablation space as new devices and heart imaging technology hit the market.
By Susan Kelly • Nov. 18, 2024 -
Haemonetics to close California facility, lay off 75 people
Over the past year, Haemonetics has reshaped its product portfolio through restructuring and M&A, adding devices used in cardiology procedures.
By Susan Kelly • Nov. 14, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
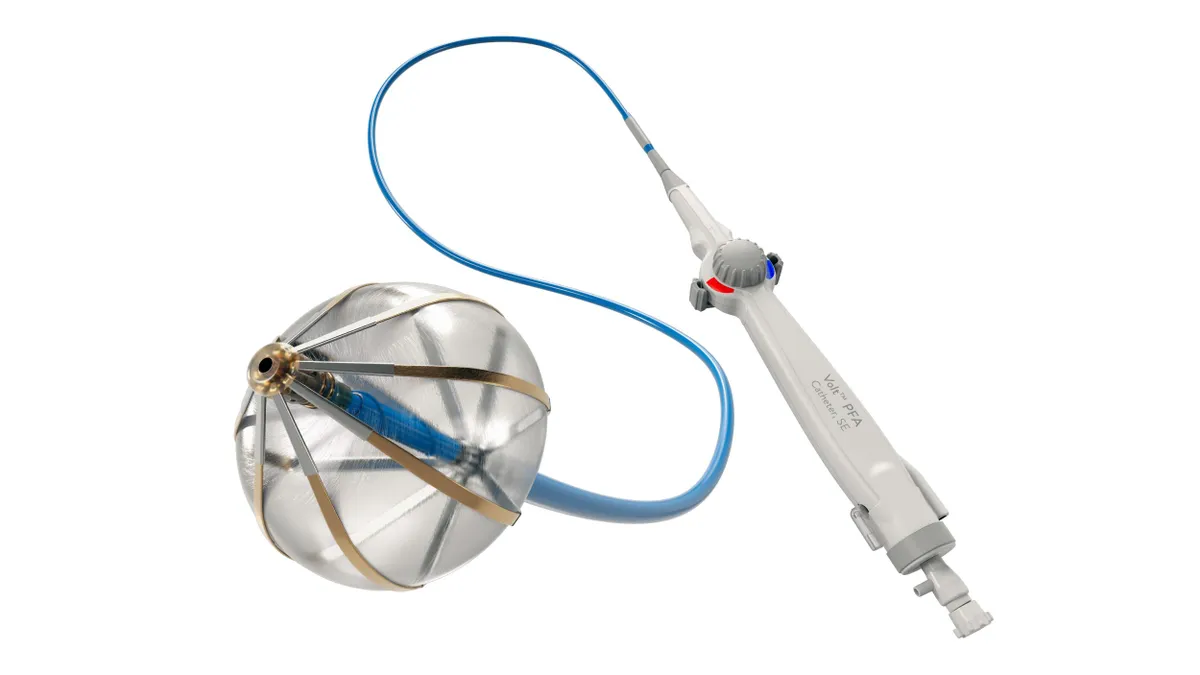
Boston Scientific resumes PFA trial in new patient group after pausing enrollment
Boston Scientific expects to complete enrollment in the coming months in the study comparing pulsed field ablation to anti-arrhythmic drugs in patients with persistent AFib, a spokesperson said.
By Susan Kelly • Nov. 12, 2024 -
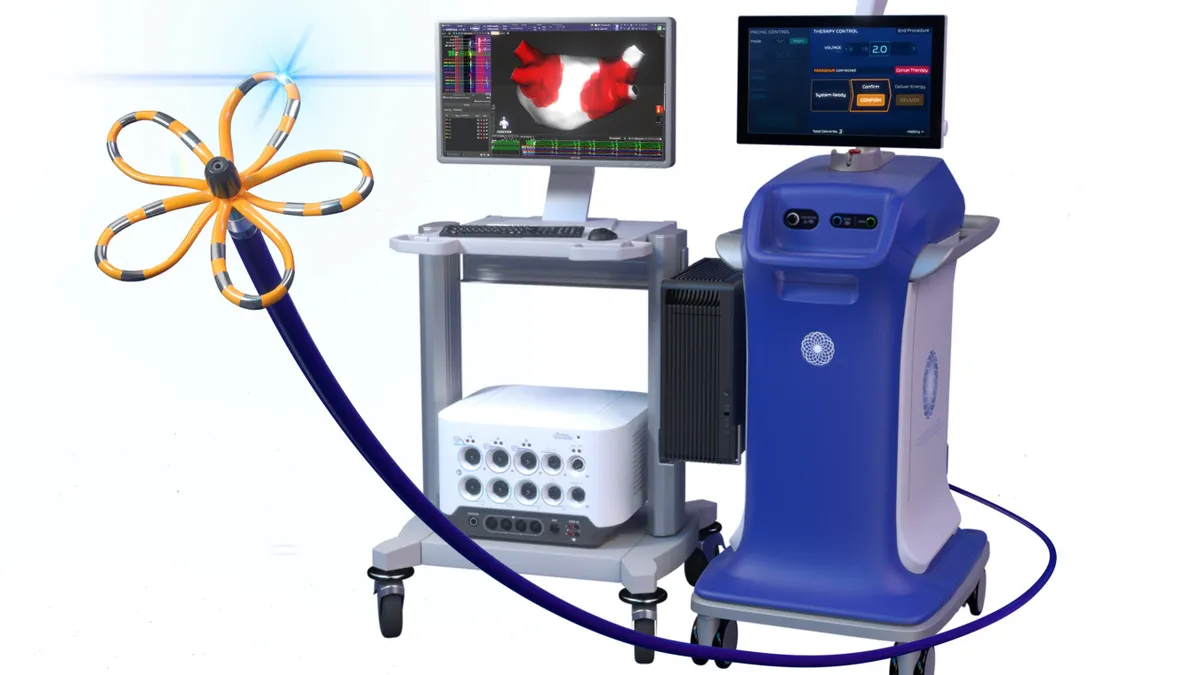
J&J wins FDA approval for Varipulse PFA system
J&J is the third medtech company to gain U.S. approval for pulsed field ablation, a new atrial fibrillation treatment seeing rapid adoption.
By Susan Kelly • Nov. 7, 2024 -
Medtronic, Recor win CMS transitional payment coverage for renal denervation devices
Patient and physician education is the next challenge to change the treatment paradigm for drug-resistant hypertension, Citi Research analyst Joanne Wuensch said.
By Susan Kelly • Nov. 5, 2024 -
Edwards, Medtronic and Inari share trial data at TCT conference
The three medtech firms unveiled new data this week during TCT 2024 in Washington, D.C. Here’s what the results mean for the companies and their device markets.
By Nick Paul Taylor • Oct. 31, 2024 -
Boston Scientific’s Acurate Neo2 inferior to rival TAVR valves in study
Analysts said the findings complicate the outlook for a U.S. launch of Boston Scientific's aortic valve replacement device.
By Susan Kelly • Oct. 31, 2024 -
Edwards data show benefit of early TAVR over ‘watchful waiting’
Study author Philippe Genereux said the trial data “shatter 60 years of ingrained belief on the treatment for severe aortic stenosis.”
By Susan Kelly • Oct. 29, 2024 -
J&J to seek FDA approval after small-bore Impella heart pump hits trial goal
The company has predicted the narrower Impella ECP will be easier to insert and implant, as well as enable the use of small bore access and closure techniques.
By Nick Paul Taylor • Oct. 29, 2024 -
Boston Scientific to close Silk Road Medical headquarters, lay off 138 people
Following its acquisition of Silk Road in September, Boston Scientific said it plans to consolidate the company’s work in Minnesota.
By Elise Reuter • Oct. 28, 2024 -
Edwards’ TAVR sales faced ongoing hospital constraints in Q3
Hurt by a slowdown in procedures for its heart valve replacement devices, Edwards said it is working with hospitals to manage workflow challenges.
By Susan Kelly • Oct. 25, 2024 -
Medtronic wins FDA approval for Affera mapping and ablation system
Affera combines mapping technology with a catheter capable of performing radiofrequency and pulsed field ablation.
By Nick Paul Taylor • Oct. 25, 2024 -

 Retrieved from iRhythm on October 22, 2024
Retrieved from iRhythm on October 22, 2024
iRhythm’s Zio AT design changes win FDA clearance
The agency cleared one of two 510(k) submissions iRhythm filed for the heart monitor after receiving a warning letter from the agency last year.
By Susan Kelly • Oct. 23, 2024 -
Boston Scientific boosts PFA expectations, citing rapid Farapulse adoption
Demand for the pulsed field ablation device drove the company’s electrophysiology sales up 177% in the third quarter compared to a year ago.
By Susan Kelly • Oct. 23, 2024 -
Boston Scientific, Edwards and Dexcom usher in second week of earnings
Johnson & Johnson, Abbott and Intuitive Surgical made a mixed start to earnings season last week.
By Nick Paul Taylor • Oct. 22, 2024 -
Boston Scientific wins FDA approval for Farapulse cardiac mapping
Stifel analyst Rick Wise said Boston Scientific can now offer “one-stop-shopping” with a pulsed field ablation catheter and integrated mapping system to treat atrial fibrillation.
By Susan Kelly • Oct. 21, 2024 -
Boston Scientific blood-blocking agent tied to additional 2 deaths, 8 injuries
After a February recall, Boston Scientific has warned physicians about new safety risks for Obsidio Embolic, which is now connected to a total of 15 injuries and four deaths.
By Nick Paul Taylor • Oct. 21, 2024 -
J&J’s recent medtech buys help to prop up devices unit
Johnson & Johnson’s medtech acquisitions over the past year have fueled growth for its cardiovascular group, offsetting challenges in businesses like orthopedics and surgery.
By Ricky Zipp • Oct. 15, 2024 -
Medtronic to evaluate Affera in ventricular tachycardia
The FDA approved an early feasibility study of Medtronic’s Affera mapping and ablation system and Sphere-9 catheter in patients with ventricular tachycardia, an abnormal heart rhythm.
By Susan Kelly • Oct. 15, 2024 -
J&J, Abbott and Intuitive kick off latest medtech earnings season
From pulsed field ablation to diabetes tech and surgical robots, the updates will provide an early look at how key medtech markets performed last quarter.
By Nick Paul Taylor • Oct. 14, 2024 -
Abbott enrolls pivotal PFA study 4 months ahead of schedule
The company reported the enrollment milestone alongside news that it received clearance to sell its Advisor HD Grid X Mapping Catheter and started a trial of its Tactiflex Duo Ablation Catheter.
By Nick Paul Taylor • Oct. 11, 2024 -
Elucid wins FDA 510(k) nod for heart plaque image analysis software
The software turns coronary CT angiography images into 3D models that quantify and classify plaque morphology to improve predictions of heart attack and stroke risk.
By Nick Paul Taylor • Oct. 4, 2024 -
FDA authorizes Pi-Cardia’s valve-in-valve TAVR device
Pi-Cardia designed the Shortcut device to mitigate the risk of coronary artery obstruction by splitting aortic valve leaflets before valve placement.
By Nick Paul Taylor • Oct. 3, 2024 -
Top medtech conferences in 2025
Some of the industry’s biggest events will take place in the second half of the year, including AdvaMed’s Medtech Conference, HLTH 2025 and TCT’s annual meeting.
By Ricky Zipp • Updated June 30, 2025 -
Recalled heart devices had limited clinical testing, study finds
Just 30 of 157 heart devices with Class I recalls underwent premarket clinical testing, according to a study published in the Annals of Internal Medicine.
By Elise Reuter • Sept. 24, 2024