Medical Devices: Page 102
-
FDA floats independent contractor to track MDUFA hiring following medtech pressure
The agency made the offer during the Medical Device User Fee Amendments V meetings in response to industry negotiators who want a clearer picture of how the program uses the money it receives.
By Nick Paul Taylor • Dec. 9, 2021 -
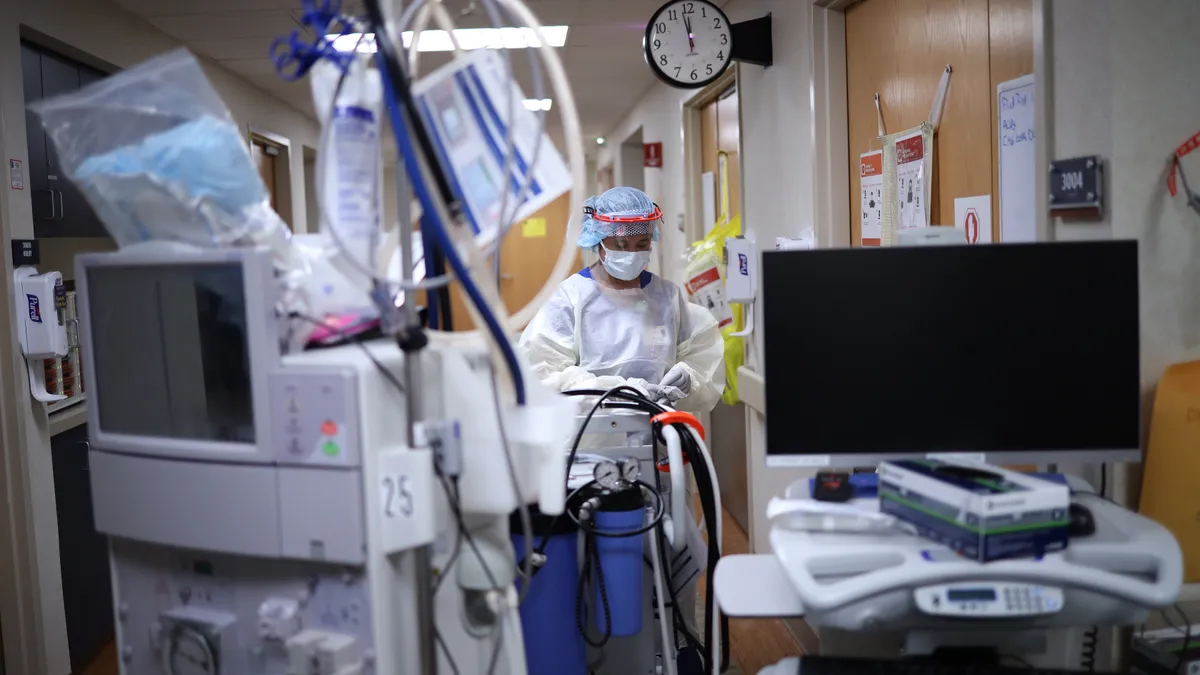
Study finds surgery volumes bounced back after 2020 COVID-19 shutdown, confirming medtech sales trends
Confounding factors such as healthcare staffing shortages caused the delta variant headwind to drag on — and, now, rapidly increasing inpatient volumes are leading U.S. hospitals to once again delay surgical procedures.
By Nick Paul Taylor • Dec. 9, 2021 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Study links cochlear implants to new bone formation, long-term residual hearing loss
Ultra-high spatial resolution CT detected new bone formation in two-thirds of the patients within four years of implantation, which adversely affected their long-term hearing preservation, according to a study in the journal Radiology.
By Nick Paul Taylor • Dec. 8, 2021 -
Implantable cardioverter defibrillators are underused in women, racial minorities: study
The proportion of people who received an ICD rose from 11.6% in 2003 to 17% in 2014, according to the Mayo Clinic study. However, that overall figure masks differences between patient groups.
By Nick Paul Taylor • Dec. 8, 2021 -
Tandem Diabetes Care targets 1M customers by 2027
The insulin pump maker expects to triple its installed units over the next five years behind new product launches and capturing market share as use among Type 1 and Type 2 patients is projected to take off.
By Ricky Zipp • Dec. 7, 2021 -
Cancer treatments lead latest FDA breakthrough device designations
The agency granted regulatory privileges to therapies for lung cancer and bone metastases from RefleXion Medical and Zetagen Therapeutics, respectively.
By Nick Paul Taylor • Dec. 7, 2021 -
Insulet Omnipod 5 release
Insulet's Omnipod 5 insulin pump cleared by FDA after months of delays, sending shares up 9%
The clearance better positions Insulet to compete with rival Tandem Diabetes Care, and comes while Medtronic's diabetes group is managing multiple product safety problems.
By Susan Kelly , Ricky Zipp • Updated Jan. 28, 2022 -

 Retrieved from Adobe Stock.
Retrieved from Adobe Stock. Sponsored by Avail Medsystems
Sponsored by Avail MedsystemsCan medical device reps have a healthy work-life balance and a growing career? Yes and yes.
Medical device reps face long work days and extensive travel schedules, but technology is changing the way they do business.
Dec. 6, 2021 -
Device recalls jumped 36% in Q3, first quarterly increase since 2020: report
The primary reasons for recalls in the quarter were software issues, which have been the leading causes in 21 of the last 22 quarters, according to Sedgwick's November U.S. product recall index.
By Ricky Zipp • Dec. 3, 2021 -
NuVasive resumes US shipments of titanium-based orthopaedic devices, gets FDA thumbs up
The agency voiced its support for the reintroduction of the Precice products, telling healthcare providers that the availability of the devices is in the best interests of patients as the benefits outweigh the known risks.
By Nick Paul Taylor • Dec. 2, 2021 -
EU finalizes implementing regulation for Eudamed medical device database
The European Commission has provided a framework for the basic operation of the system, slated for a May 2022 launch, including how to access it, what it will do in the event of a database malfunction and IT security measures.
By Nick Paul Taylor • Dec. 2, 2021 -
Deep Dive
Medical device security continues to be casualty of hospital-medtech divide
FDA says manufacturers and hospitals are both responsible for protecting devices from growing cybersecurity threats. However, experts say healthcare providers carry a much heavier load.
By Greg Slabodkin • Dec. 1, 2021 -
Cyber playbook sets out strategies for modeling threats to medical devices
The FDA-funded guide arrives against a backdrop of calls from the agency for the medtech industry to step up its threat modeling throughout the device lifecycle in order to strengthen cybersecurity and patient safety.
By Nick Paul Taylor • Dec. 1, 2021 -
FDA updates eSTAR ahead of expanding filing template for De Novo submissions
The platform has been available for manufacturers to voluntarily submit 510(k) submissions since September 2020. The agency will start accepting De Novo applications when a final rule takes effect early next year.
By Nick Paul Taylor • Nov. 30, 2021 -
4 takeaways from Medtronic's latest earnings report
The medtech giant on Tuesday discussed a wide range of issues impacting the company, from whether procedure volumes rebounded in November to the chances of it spinning off non-core or underperforming business units.
By Nick Paul Taylor • Nov. 24, 2021 -
Roundup: COVID-19 surge, hospital labor shortages, supply chain issues hit medtechs in latest earnings
After the industry began recovering in the first half of the year, top companies reported that the delta variant surge put a drag on businesses last quarter.
Nov. 23, 2021 -
Medtronic blames revenue miss on COVID-19 resurgence, hospital staffing shortages
CEO Geoff Martha said the medtech was hit in its second quarter by pandemic-related challenges that weighed on procedure volumes, particularly in the U.S.
By Greg Slabodkin • Nov. 23, 2021 -
FDA resumes domestic surveillance inspections as omicron cases decline
The regulator has restarted the examinations, following a six-week freeze in response to the variant's surge. FDA plans to conduct foreign prioritized inspections starting in April.
By Nick Paul Taylor • Updated Feb. 7, 2022 -
Rise of TAVR increased overall aortic valve replacement, improved outcomes: study
The growth of transcatheter procedures has driven a 60% increase in aortic valve replacement and cut one-year mortality in older adults, according to data published in the Journal of the American College of Cardiology.
By Nick Paul Taylor • Nov. 23, 2021 -
Latest breakthrough device designations go to brain-computer interface, exo-suit
Regulatory privileges were awarded to Blackrock Neurotech's brain-computer interface, designed to assist immobile patients with performing activities, and ReWalk Robotics' exo-suit, which is intended to help stroke patients walk.
By Nick Paul Taylor • Nov. 22, 2021 -
Q&A
New iRhythm CEO talks Medicare pricing, larger medtech competitors, international markets
"I think the near-term noise is something that we'll work through, but the underlying technology has never been questioned," Quentin Blackford told MedTech Dive.
By Ricky Zipp • Nov. 19, 2021 -
Stryker dismisses robotics threat from J&J, Zimmer with bullish forecast
CEO Kevin Lobo said during Thursday's analyst day that the company is "poised and ready to pounce" if valuations fall as Stryker looks to leverage M&A in long-term growth plans.
By Nick Paul Taylor • Nov. 19, 2021 -
Medtronic leadless pacemaker flagged by FDA for safety risks
The agency said in a letter to healthcare providers that cardiac perforations associated with Medtronic's Micra device are more likely than traditional pacemakers to be associated with serious complications, such as death.
By Nick Paul Taylor • Nov. 18, 2021 -
TAVR uptake in US cities tied to race, ethnicity and income: JAMA study
ZIP codes with higher proportions of Black, Hispanic and socioeconomically disadvantaged patients have lower rates of transcatheter aortic valve replacement procedures, according to an analysis of Medicare claims data.
By Nick Paul Taylor • Nov. 18, 2021 -
MCIT breakthrough device payment pathway included in Cures 2.0 bill
Lawmakers on Tuesday introduced a revised MCIT proposal in the next version of the 21st Century Cures Act, following last week's repeal of the final rule by CMS. Industry groups AdvaMed and MDMA both back the legislation.
By Nick Paul Taylor • Nov. 17, 2021