Medical Devices: Page 139
-
 National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from https://www.flickr.com/photos/nihgov/49565662436/in/album-72157713108522106/.
National Institute of Allergy and Infectious Diseases. (2020). "Novel Coronavirus SARS-CoV-2" [Image]. Retrieved from https://www.flickr.com/photos/nihgov/49565662436/in/album-72157713108522106/.
Diagnostic, device makers scrambling as COVID-19 sweeps US
What began as a potential Q1 headwind for medtechs manufacturing in China has quickly turned into a global crisis with unprecedented challenges and opportunities as the sector grapples with the pandemic.
March 20, 2020 -
Medtronic pledges '24/7' ventilator production to meet coronavirus demand
The company is aiming to more than double its manufacturing capacity as GE, Getinge, Philips and others outside of healthcare commit to boosting worldwide supply of the potentially lifesaving machines.
By Maria Rachal • March 19, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
CMS urges hospitals to put off elective procedures
The guidelines suggest three tiers, with procedures like hip and knee replacements falling into the "consider postponing" category.
By Shannon Muchmore • March 19, 2020 -
New notified bodies trickle in ahead of looming MDR start date
The 12th notified body authorized for MDR work, and the first from Hungary, was listed in the NANDO database Friday: CE Certiso.
By Nick Paul Taylor • Updated March 20, 2020 -
FDA pulls plug on routine US inspections
The move to protect staff and acknowledge industry worries about outside visitors amid coronavirus comes a week after the agency said it would postpone nearly all overseas inspections through April.
By Nick Paul Taylor • March 19, 2020 -
Lessons from Hurricane Maria could aid Baxter's coronavirus operations
The aftermath of the 2017 storm led to serious manufacturing disruptions to Baxter's IV business and prompted it to strengthen its supply network, which CEO Joe Almeida said might be abating challenges with COVID-19 so far.
By Maria Rachal • March 18, 2020 -
Moody's cuts medtech outlook on coronavirus upheaval
The pandemic is likely to stall significant M&A activity in the sector, the ratings agency predicts.
By Susan Kelly • March 18, 2020 -
MedTech Europe sounds alarm over 'inconsistent' enforcement amid flurry of MDR updates
A lack of harmonized standards worries the trade association. Meanwhile, the Medical Device Coordination Group issued new documents on software requirements, implant cards and unique device identifiers.
By Nick Paul Taylor • March 18, 2020 -
Intuitive Surgical among 1st big medtechs to warn of costly pandemic disruptions
Delayed placements and procedures in China and Italy may not have a material impact on the robotic surgery giant, but similar trends in the U.S. and Europe, along with reduced capital spending by hospitals, present a greater threat.
By Maria Rachal • March 17, 2020 -
Endologix secures FDA approval for new abdominal stent graft
With the launch of its Alto graft device platform, the company hopes to rebuild after a series of setbacks with earlier versions of its abdominal aortic aneurysm, or AAA, repair technology.
By Susan Kelly • March 17, 2020 -
As coronavirus roils region, EU device body adamant about steps to hit MDR deadline
The Medical Device Coordination Group acknowledged implementation “has proven to be a very challenging task” but for now appears to be pushing ahead with the planned May start date.
By Nick Paul Taylor • March 17, 2020 -
Value-based payment models take hold in medtech, but barriers slow shift
The arrangements are becoming more prevalent, especially for costly devices used in chronic disease management and in hospital settings, according to a recent Deloitte report.
By Susan Kelly • March 16, 2020 -
Hospitals advised to rethink elective procedures, potentially straining device makers
The Surgeon General and American College of Surgeons urged hospitals to "minimize, postpone, or cancel" certain procedures while handling the COVID-19 outbreak, presenting at least a temporary risk to medtechs' revenue.
By Amritpal Sandhu-Longoria, Maria Rachal • March 16, 2020 -
Senseonics cites Freestyle Libre pricing as factor in its Roche deal challenges
The implantable continuous glucose monitor maker forecast sales outside the U.S. to drop by one-third this year.
By Nick Paul Taylor • March 13, 2020 -
TransMedics stock falls double-digits as PMA meeting again delayed by FDA
The "unforeseen delay," according to the company's CEO, further pushes back the advisory panel review of the company's heart transplant device.
By Maria Rachal • Updated Sept. 29, 2020 -
FDA finalizes contentious guidance on third party 510(k) reviews
Meant to enable faster decisions and free up the agency to focus on higher risk devices, earlier versions took fire from AdvaMed and some patient groups.
By Nick Paul Taylor • March 12, 2020 -
Rival to Boston Scientific device used in TAVR wins CE mark
Keystone Heart, the Israel-based unit of Chinese structural heart player Venus Medtech, is readying an FDA submission for its cerebral embolic protection device, intended to reduce stroke risk during transcatheter heart procedures.
By Susan Kelly • March 11, 2020 -
Medtronic's HVAD controversy
FDA issues Class I recall for Medtronic's HeartWare HVAD after patient death
A design flaw flagged by Medtronic in January regarding its ventricular assist devices landed a high-risk label from regulators this week.
By Nick Paul Taylor • March 11, 2020 -
Key EU notified body meeting goes on, virtually
A Medical Device Coordination Group meeting, parts of which are postponed, comes as Germany, home to four of 11 notified bodies designated under EU MDR, anticipates widespread COVID-19 transmission.
By Nick Paul Taylor , Maria Rachal • March 11, 2020 -
FDA updates guidance on 510(k) submissions for electrosurgical devices
The agency offered greater detail on testing requirements in assessing thermal tissue damage.
By Susan Kelly • March 10, 2020 -
ECRI: Poor sterilization, failure to learn from device problems threaten patient safety
The 2020 list put together by the nonprofit ECRI Institute also highlights diagnostic errors as a top patient safety concern for a third consecutive year.
By Maria Rachal • March 10, 2020 -
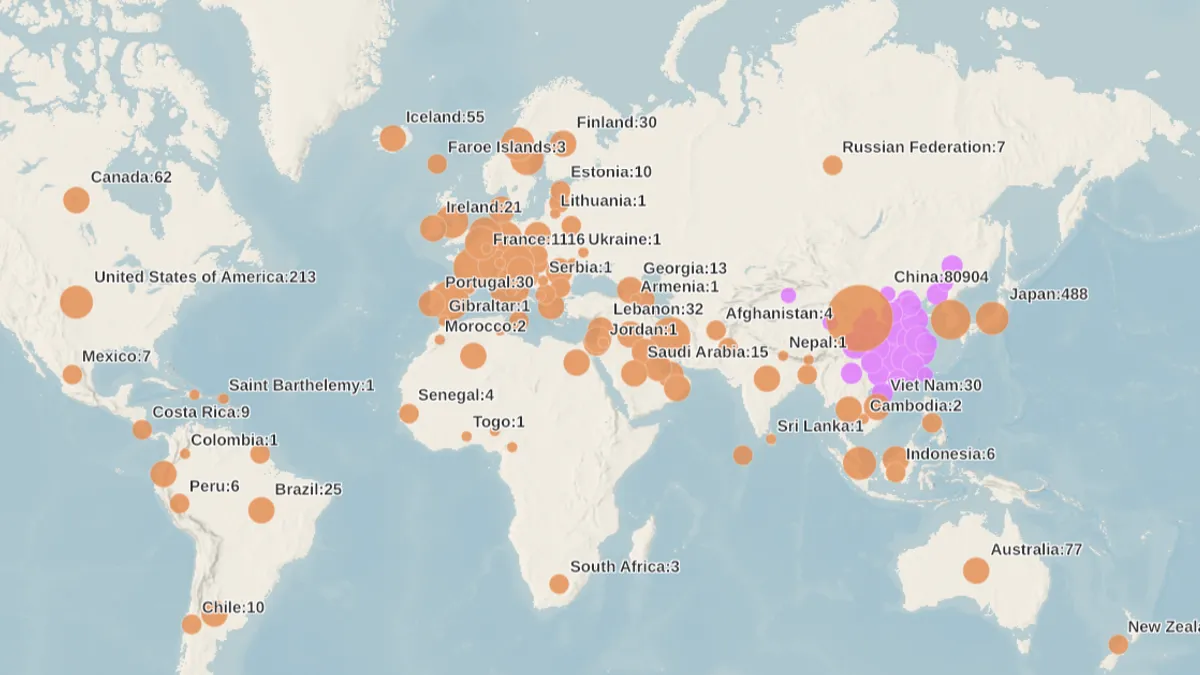
 World Health Organization. (2020). "Map of impact" [Photo]. Retrieved from https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd.
World Health Organization. (2020). "Map of impact" [Photo]. Retrieved from https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd.
Hospitals dipping into emergency stocks to combat coronavirus
As more community spread of COVID-19 is reported daily, hospitals are bracing for a rush of patients and payers are waiving fees for telemedicine consults and extra prescription drug supplies.
By Shannon Muchmore • March 10, 2020 -
Insightec nets up to $150M for incisionless brain surgery tech
Backed by Koch Industries, the privately held medtech makes non-invasive technology that uses ultrasound to help treat ailments such as essential tremor and Parkinson’s disease.
By Greg Slabodkin • March 9, 2020 -
TMS pioneer Neuronetics gets breakthrough nod, CEO departs
The struggling neuromodulation device maker said it will get an expedited FDA review in pursuit of an additional indication for its transcranial magnetic stimulation system in people with bipolar depression.
By Susan Kelly • March 9, 2020 -
FDA gives Class I label to BD Alaris infusion pump system recall
The regulatory agency reported 55 injuries and one death, with 774,000 devices impacted in U.S.
By Greg Slabodkin • March 6, 2020





