Medical Devices: Page 42
-
 Retrieved from Food and Drug Administration.
Retrieved from Food and Drug Administration.
Cardinal receives FDA warning letter over unapproved syringes
The agency has recently increased scrutiny of plastic syringes made in China because of safety concerns, including blocking imports from two Chinese manufacturers.
By Elise Reuter • May 2, 2024 -
Philips reaches major settlements related to respiratory devices recall
A judge recently finalized an economic claims settlement, and Philips said it reached an agreement on medical claims.
By Elise Reuter • May 2, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medline to buy Ecolab’s surgical products for nearly $1B
The medical products supplier will acquire Ecolab’s sterile drapes and fluid temperature management systems.
By Susan Kelly • May 1, 2024 -
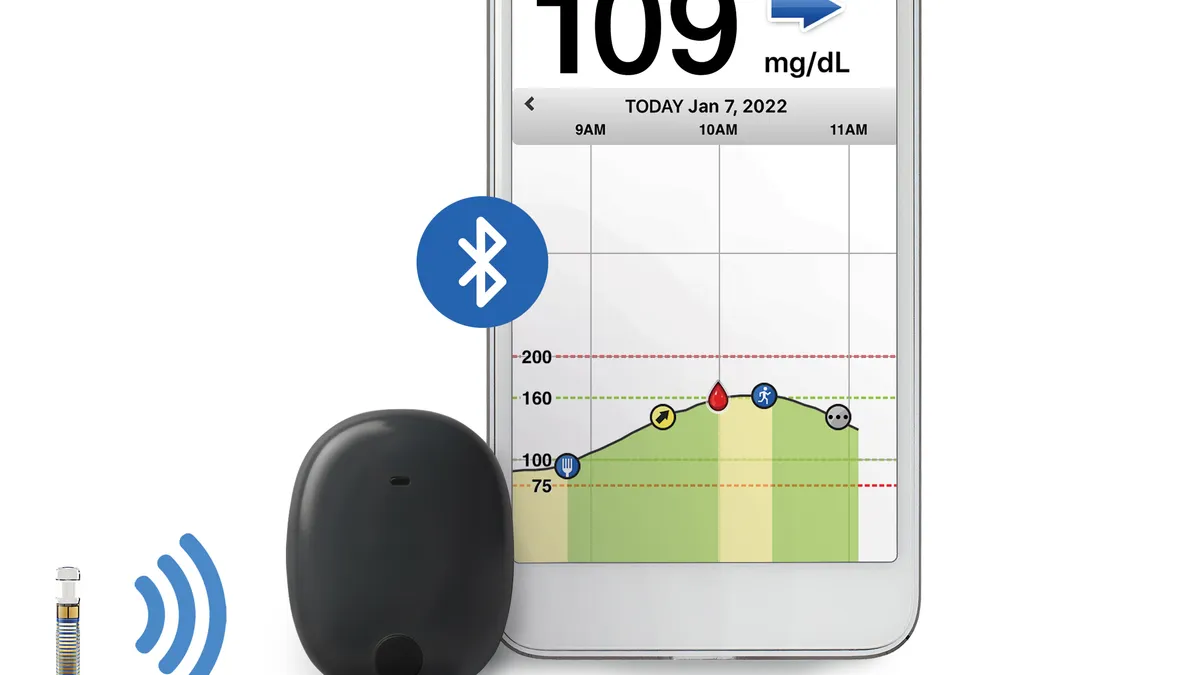
Senseonics gets FDA clearance to pair CGM implant with insulin pumps
Analysts said the designation and planned one-year sensor could help Senseonics’ device appeal to more patients.
By Nick Paul Taylor • May 1, 2024 -
Hologic to buy Endomagnetics for $310M
Endomagnetics, which makes guidance technology for breast cancer surgery, brought in about $35 million in revenue last year.
By Elise Reuter • April 30, 2024 -
Abbott wins FDA approval for Esprit resorbable scaffold
While RBC analysts wrote there’s “strong appetite” for the system, BTIG said the need to train centers could result in a gradual launch.
By Nick Paul Taylor • April 30, 2024 -
Medtronic to lay off 44 employees in California
Medtronic’s latest layoff plan adds to what has become a long list of job cuts for the medical device industry this year.
By Ricky Zipp • April 29, 2024 -
Philips agrees to $1.1B settlement of US respiratory recall lawsuits
CEO Roy Jakobs said on a first-quarter earnings call that “for the U.S., this is as final as it can get.”
By Nick Paul Taylor • April 29, 2024 -
Medtronic wins FDA approval for Inceptiv closed-loop spinal cord stimulator
Evercore analysts said the launch could help Medtronic regain market share in the coming quarters.
By Nick Paul Taylor • April 29, 2024 -
Fresenius Medical Care recalls 2M dialysis devices over toxin exposure risk
Nearly 2.2 million devices are affected by the recall. Fresenius Medical Care will not have to remove products due to the issue.
By Nick Paul Taylor • April 26, 2024 -
Edwards gets Q1 boost as Pascal valve repair sales ramp up
New mitral and tricuspid valve treatments are attracting strong demand, but analysts said transcatheter aortic valve replacement sales were shy of expectations.
By Susan Kelly • April 26, 2024 -
Infutronix infusion pump recall linked to 6 injuries, 1 death
The FDA’s recall notice sheds light on the impact to patients weeks after the company told customers it would pull Nimbus pumps from the market.
By Nick Paul Taylor • April 26, 2024 -
Judge approves settlement of more than $500M from Philips recall
Philips could be on the hook for more costs if eligible claims for recalled or returned respiratory devices exceed the amount agreed to in the settlement.
By Elise Reuter • April 25, 2024 -
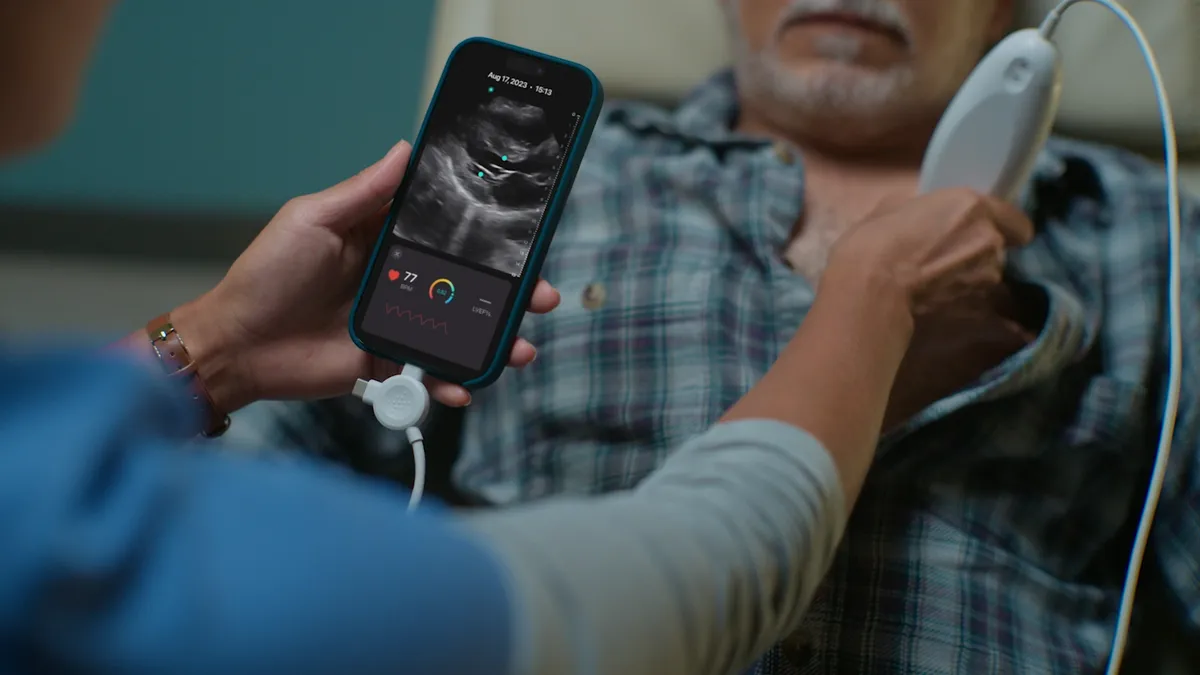
Exo adds FDA-cleared AI tools to handheld ultrasound system
Exo sees the new capabilities as especially helpful for healthcare providers in rural and under-resourced settings.
By Nick Paul Taylor • April 25, 2024 -
Philips failed to report corrections of CT machines, FDA says in warning letter
After a 2023 inspection, the FDA found three unreported field corrections related to CT machines and 19 other recalls of radiology devices.
By Elise Reuter • April 25, 2024 -
Medtech’s top executive moves in 2024
Recent executive changes include former Medtronic CFO Karen Parkhill leaving for HP and Masimo CEO Joe Kiani resigning. Check out MedTech Dive’s roundup of leadership movement in 2024.
By Susan Kelly , Ricky Zipp • Updated Sept. 26, 2024 -
EU launches probe of China’s medical device market
China’s procurement market for medical devices has gradually become more closed for European and foreign companies, the European Commission alleges.
By Susan Kelly • April 24, 2024 -
Medtronic names Yarmela Pavlovic as chief regulatory officer
Pavlovic, who was a partner at the law firm Manatt, Phelps & Phillips before joining Medtronic, will gain a new title, but her role will stay the same.
By Nick Paul Taylor • April 24, 2024 -
Boston Scientific gives first look at Farapulse US rollout
CEO Mike Mahoney would not provide specific sales numbers for the device, but hyped the pulsed field ablation system as the “most transformational product” he’s seen in his time with the company.
By Ricky Zipp • April 24, 2024 -
GE Healthcare subsidiary expands radiation therapy partnership with Elekta
MIM Software, which GE Healthcare acquired earlier this month, will work with Elekta to improve treatment planning and workflows.
By Elise Reuter • April 23, 2024 -
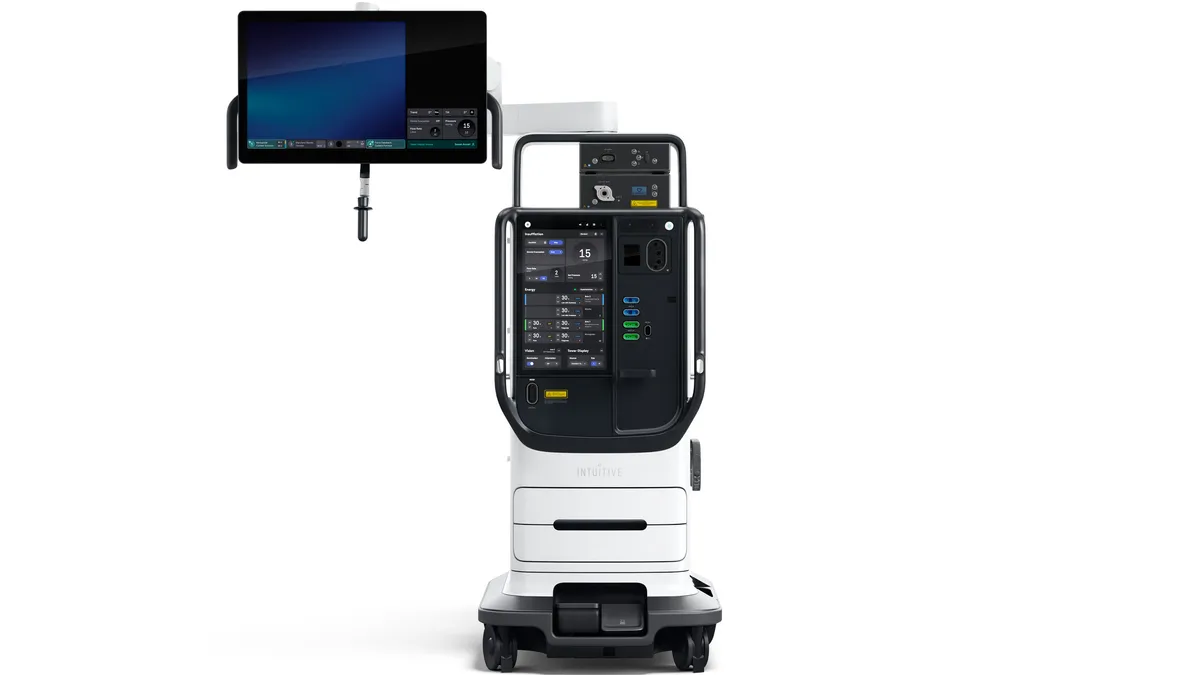
Intuitive’s da Vinci 5 robot could widen ‘already substantial’ lead: analysts
Truist Securities analysts wrote that Medtronic and Johnson & Johnson could “be relegated to niche players” in surgical robotics.
By Nick Paul Taylor • April 23, 2024 -
Boston Scientific, Dexcom and Edwards next as earnings season rolls on
J.P. Morgan analysts expect this week’s results to be “very good” following reports from J&J, Abbott and Intuitive Surgical.
By Nick Paul Taylor • April 23, 2024 -
FDA approves Lumicell’s breast cancer imaging tool
Lumicell developed the technology to enable physicians to detect residual cancer in the breast cavity after surgery.
By Nick Paul Taylor • April 22, 2024 -
Exactech recalls shoulder devices after initially declining to act
After the FDA issued a public safety notice and sent Exactech a warning letter, the firm agreed to recall shoulder implants due to packaging issues.
By Nick Paul Taylor • April 22, 2024 -
Intuitive’s rollout of new da Vinci 5 robot steals Q1 spotlight
While Intuitive has already placed eight new surgical robots, executives cautioned system placements could be choppy in the coming months.
By Susan Kelly • April 19, 2024