Medical Devices: Page 43
-
Spinoffs, sales and tuck-ins top medtech deal priorities: Moody’s
After J&J’s $13.1 billion purchase of Shockwave Medical, Moody’s analysts see the potential for other larger pick-ups “if interest rates substantially decrease.”
By Nick Paul Taylor • April 19, 2024 -
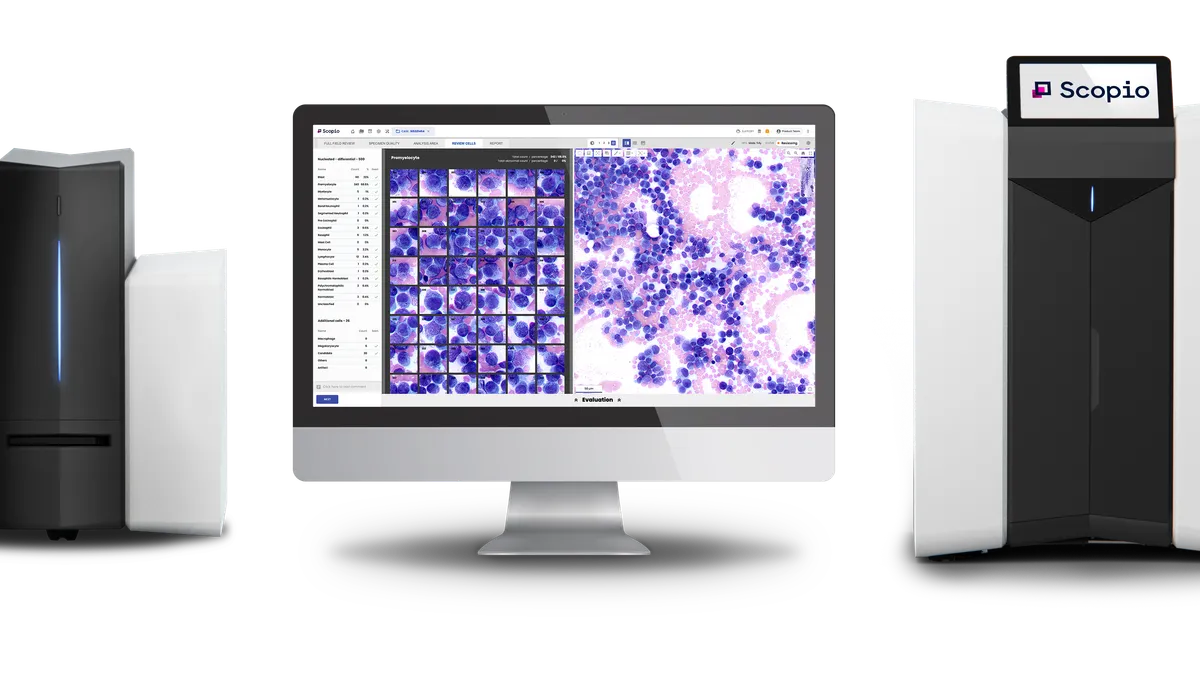
Scopio Labs wins de novo nod for bone marrow analysis software
Scopio can now add the application to its imaging platforms that allow users to view blood samples digitally rather than under a microscope.
By Nick Paul Taylor • April 18, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
23andMe CEO plans to take company private
Anne Wojcicki wants to buy all outstanding shares of the DNA testing firm, which has seen its stock slump in recent years.
By Elise Reuter • April 18, 2024 -
Boston Scientific recalls blood-blocking agent linked to 2 deaths
Seven injuries and 11 incidents were also associated with the safety issue.
By Nick Paul Taylor • April 18, 2024 -
‘An incredible undertaking’: 5 takeaways from Philips consent decree
Under the agreement, the FDA will use rare powers to require repairs, replacements or refunds for recalled respiratory machines.
By Elise Reuter • Updated April 17, 2024 -
Abbott looks to ‘highly productive’ device pipeline for future growth
CEO Robert Ford highlighted new and upcoming products throughout the earnings call, calling the recently approved Triclip valve a “billion-dollar opportunity."
By Elise Reuter • April 17, 2024 -
FDA warns Soulaire to stop selling device outside authorized uses
Soulaire marketed its external counterpulsation system to grow new arteries, repair organ dysfunction and treat COVID-19, among other uses, without evidence.
By Nick Paul Taylor • April 17, 2024 -
GE Healthcare imaging CEO to resign
Jan Makela plans to leave the company in July to join another firm.
By Elise Reuter • April 16, 2024 -
‘We think in decades’: J&J’s $30B spending spree may not be over
After Johnson & Johnson has doled out more than $30 billion for Abiomed, Laminar and Shockwave Medical, are more medtech deals on the way?
By Ricky Zipp • April 16, 2024 -
Solventum appoints Shirley Edwards to board of directors
The 3M spinoff highlighted Edwards’ expertise in mergers and acquisitions and corporate governance as an asset.
By Nick Paul Taylor • April 16, 2024 -
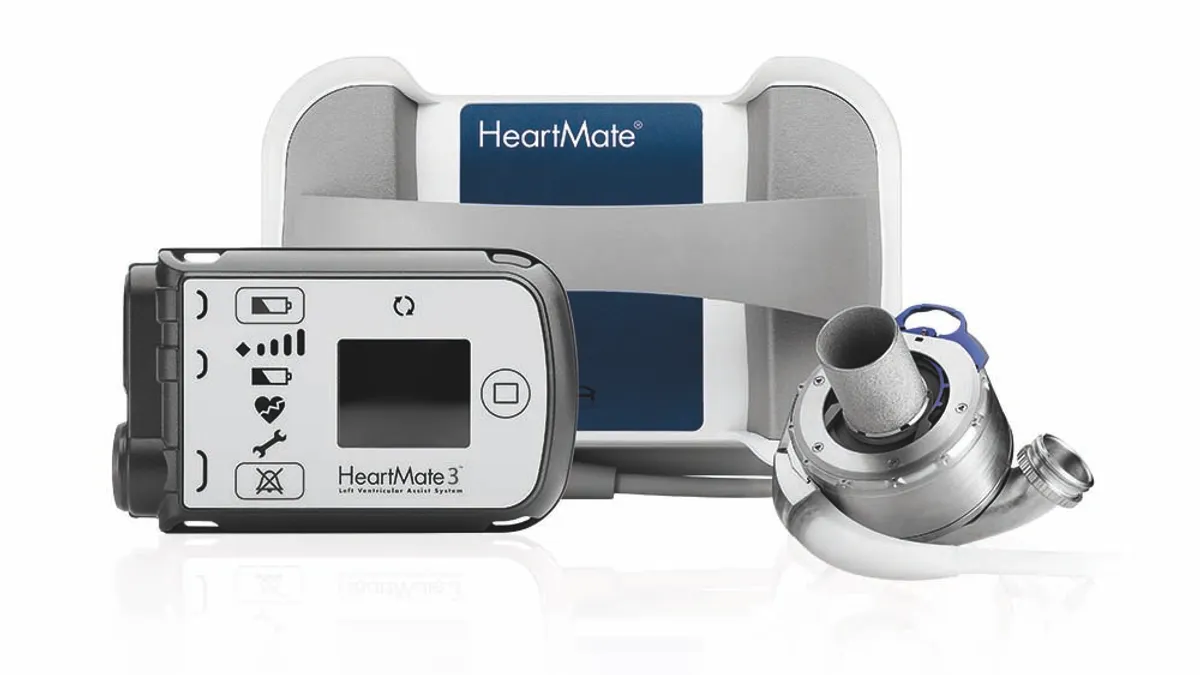
Abbott’s latest Heartmate recall tied to 273 injuries, 14 deaths
Biological material can build up and obstruct blood flow in heart failure patients supported by the left ventricular assist devices, the FDA said.
By Susan Kelly • April 15, 2024 -
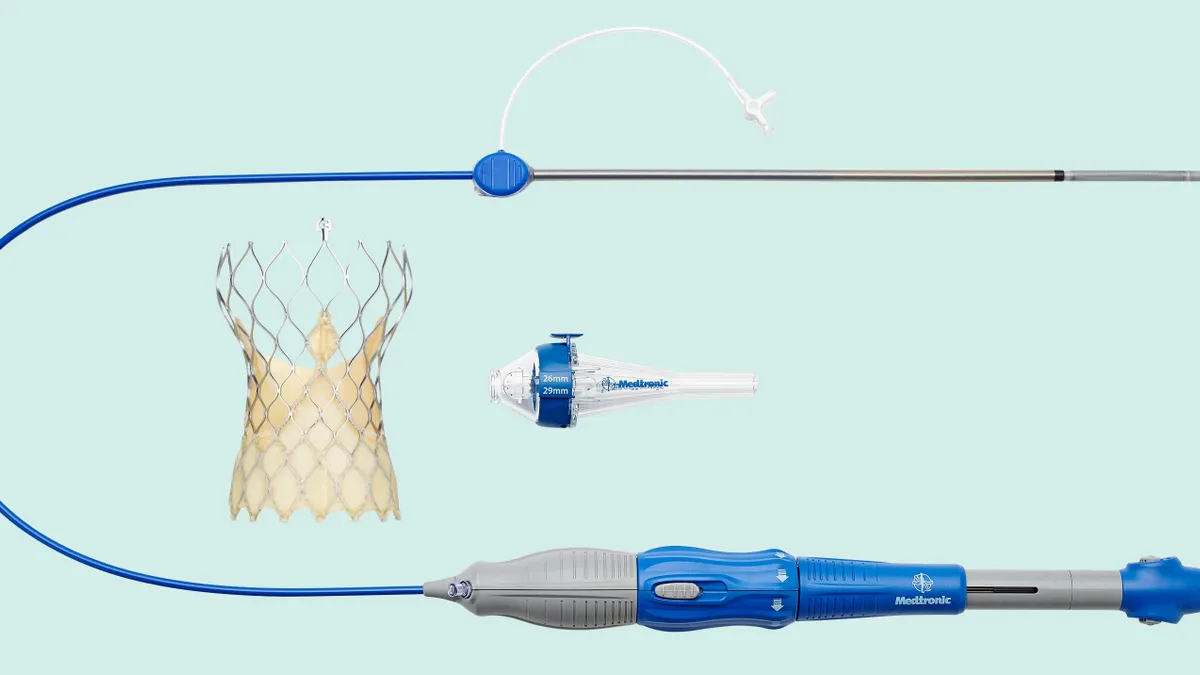
Edwards pushes back on Medtronic’s head-to-head TAVR trial
Edwards Lifesciences and Wall Street analysts questioned the results of Medtronic’s women-focused study, claiming the data are inconsistent with real-world evidence.
By Susan Kelly • April 15, 2024 -
Exact Sciences names Aaron Bloomer CFO
The executive will succeed Jeff Elliott, who said in January he would step down due to personal reasons.
By Elise Reuter • April 15, 2024 -
Cook Medical wins contract to supply implants to US Defense Department
Through the agreement, Cook will provide its Zilver PTX drug-eluting peripheral stent, Zenith aortic endografts and other implantable devices.
By Nick Paul Taylor • April 12, 2024 -
Q&A
Abbott’s Nadim Geloo on Triclip’s US launch, Edwards competition
The senior medical director for structural heart discussed the Triclip rollout, Edwards’ rival tricuspid treatment and Abbott’s aortic valve clinical trial.
By Susan Kelly • April 12, 2024 -
Osso VR brings surgery training app to Apple Vision Pro
Users can virtually walk through the steps of two orthopedic procedures: total knee replacement and carpal tunnel release.
By Nick Paul Taylor • April 12, 2024 -
Steris to sell dental segment for $787.5M
Divesting the business will allow Steris to repay debt and focus on its core markets of healthcare, pharma and medtech.
By Elise Reuter • April 11, 2024 -
FDA expands import alert to block all plastic syringes from Chinese manufacturer
The agency increased the blockade because Jiangsu Shenli Medical Production failed to meet device quality system requirements.
By Nick Paul Taylor • April 11, 2024 -
Philips restricted from selling respiratory machines in DOJ consent decree
The agreement, filed Tuesday, also has export restrictions to ensure U.S. patients receive remediated devices in a timely manner.
By Elise Reuter • April 9, 2024 -
Synchron launches patient registry to prepare for brain implant trial
The startup, which rivals Neuralink, is developing brain-computer interface technology that lets people control electronic devices hands-free.
By Elise Reuter • April 9, 2024 -
Robotic knee surgery study revives debate over procedure’s benefits
As robotic assistance gains in popularity, new research has found no advantage over the conventional approach in reducing the need for knee replacement revision surgeries.
By Susan Kelly • April 9, 2024 -
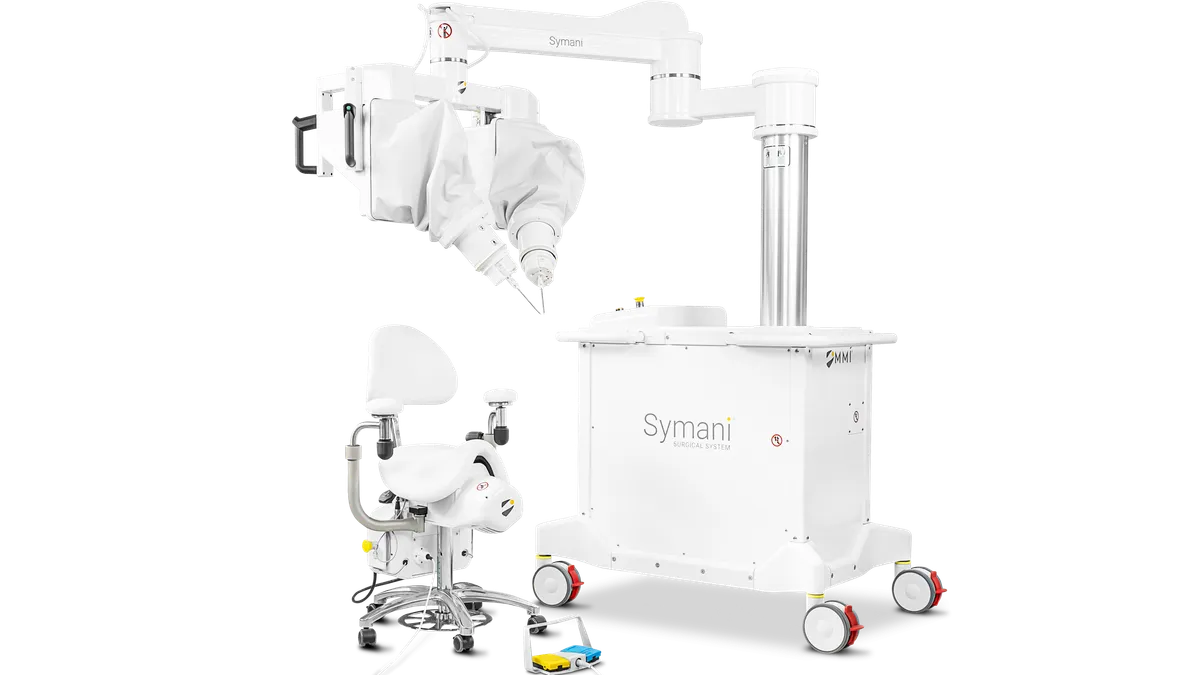
MMI receives de novo nod for microsurgery robot
Medical Microinstruments said the system could increase the number of physicians who can perform complicated microsurgical procedures.
By Nick Paul Taylor • April 9, 2024 -
Medtronic beats Edwards on valve dysfunction in TAVR study
In a head-to-head clinical trial, Medtronic linked its device to a lower rate of valve dysfunction than Edwards’ rival product in a subpopulation of patients.
By Nick Paul Taylor • April 8, 2024 -
Physicians ask FDA to revoke approval of DNA test for opioid addiction
Physicians said the test is based “on old genetic studies that have largely been abandoned” and could exacerbate the opioid crisis.
By Nick Paul Taylor • April 8, 2024 -
Beckman Coulter receives FDA warning letter
Inspectors found fault with quality practices at a facility that makes immunoassay analyzer instruments and tests.
By Nick Paul Taylor • April 5, 2024