Medical Devices: Page 79
-
Vivos bounces back from FDA rejection to land 510(k) clearance for sleep apnea treatment
Shares in Vivos increased by more than 150% on the day of the news.
By Nick Paul Taylor • Jan. 6, 2023 -
Q&A
Friday Q&A: For GE HealthCare, the future is digital, CEO Arduini says
Digital capabilities will be “the next big inflection” driving growth for GE’s newly independent healthcare business.
By Peter Green • Jan. 6, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
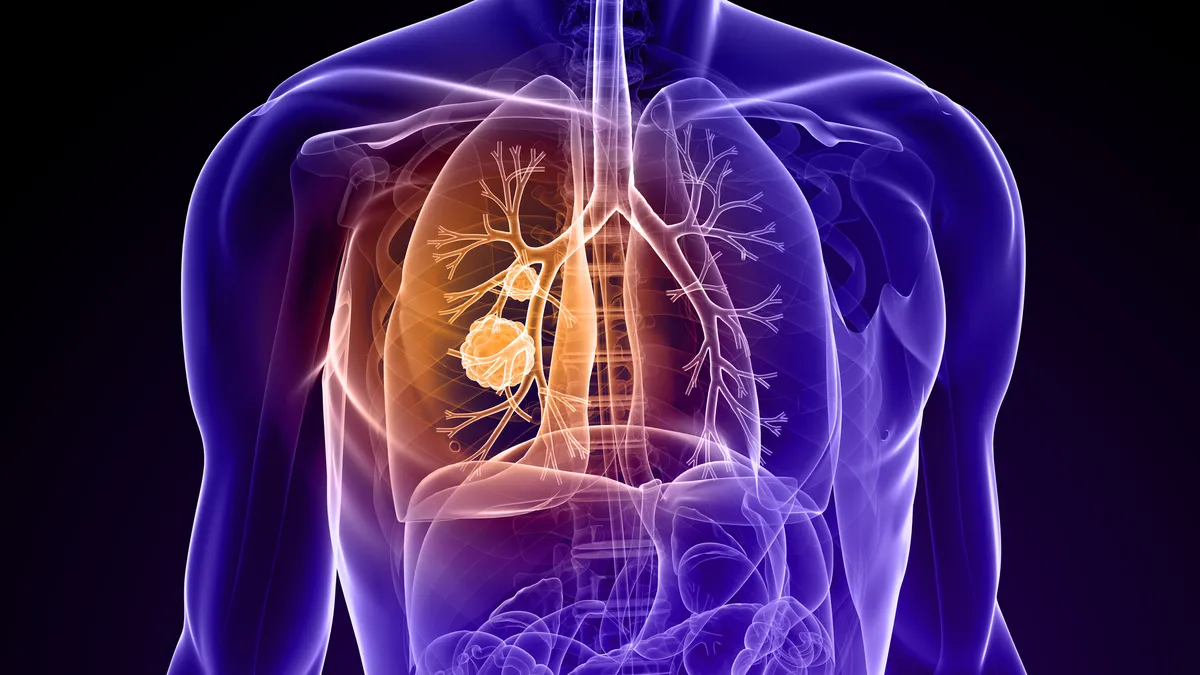
Novocure shares jump on positive lung cancer data
A regimen involving the Swiss biotech’s electrical field-based treatment extended lives in a late-stage trial. But data comparing the medical device to standard chemotherapy is less persuasive.
By Jonathan Gardner • Jan. 5, 2023 -
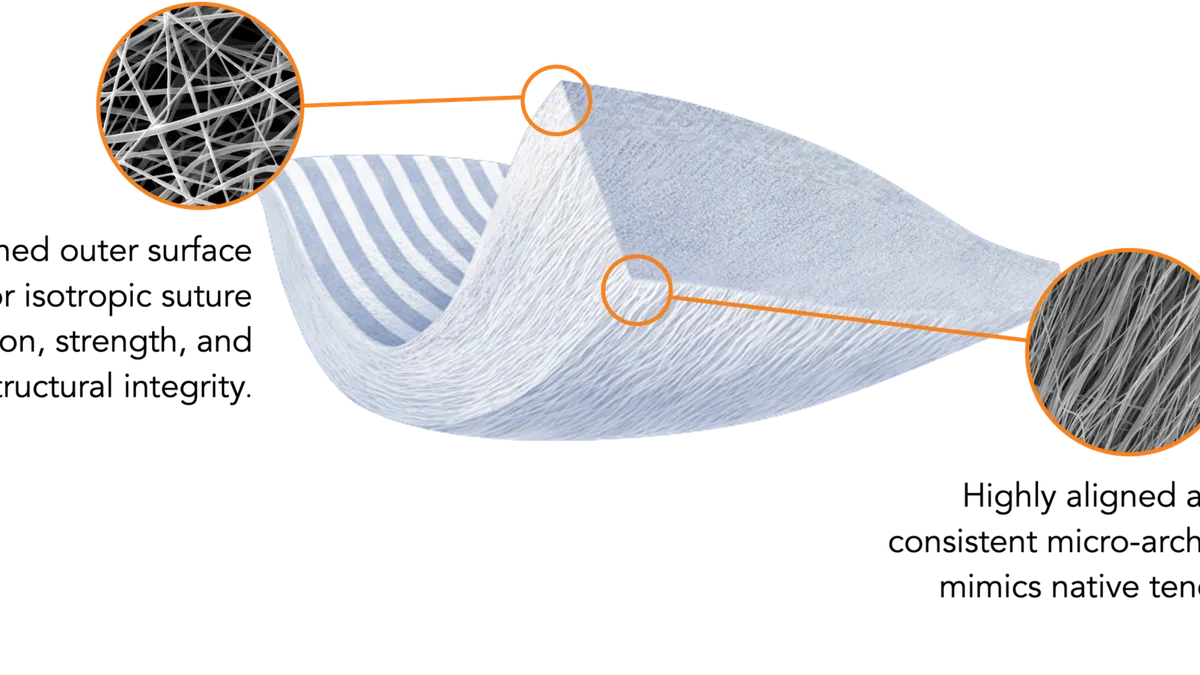
Zimmer to acquire soft tissue repair firm Embody for as much as $275M
Buying Embody’s repair system for tendons and ligaments will expand Zimmer Biomet’s share in the growing sports medicine market.
By Elise Reuter • Jan. 5, 2023 -
GE HealthCare starts trading on Nasdaq as independent company
The company’s stock gained in morning trading after debuting at $56 per share.
By Elise Reuter • Jan. 4, 2023 -
‘Mea culpa’: What Goldman Sachs analysts got wrong about Medtronic in 2022
Medtronic has struggled to deliver on key projects in recent years, leading to a decline in its stock performance.
By Nick Paul Taylor • Jan. 4, 2023 -

 Retrieved from LinkedIn on January 04, 2023
Retrieved from LinkedIn on January 04, 2023
ReCor names Healthineers exec Barghout as CEO ahead of showdown with Medtronic
ReCor is preparing to go up against Medtronic with its renal denervation system, in a market that the latter expects to grow to $3 billion by the end of the decade.
By Nick Paul Taylor • Jan. 4, 2023 -
Apple infringed on AliveCor wearable patents, ITC says in final ruling; import ban suspended
The commission found that Apple has infringed on two AliveCor patents and thereby violated a law that can trigger an import ban.
By Nick Paul Taylor • Jan. 4, 2023 -
Deep Dive
A look back at medtech’s top 10 acquisitions of 2022
While larger acquisitions were announced at the end of the year including J&J’s $16.6B purchase of Abiomed, the number of deals declined from 2021.
By Elise Reuter • Dec. 22, 2022 -
J&J closes acquisition of heart pump maker Abiomed
The $16.6 billion purchase was the largest medtech transaction of 2022.
By Elise Reuter • Dec. 22, 2022 -
Philips’ studies argue recalled DreamStation CPAP machines unlikely to harm patients’ health
In a briefing on testing of the machines, Philips said exposure to degraded foam used to soundproof the devices is unlikely to harm patients.
By Elise Reuter • Dec. 21, 2022 -
Diagnostic testing reform missing from Congress’ year-end spending bill
A provision that would have brought in-vitro diagnostic tests and lab-developed tests under one regulatory framework was not included in the omnibus spending bill.
By Elise Reuter • Dec. 21, 2022 -
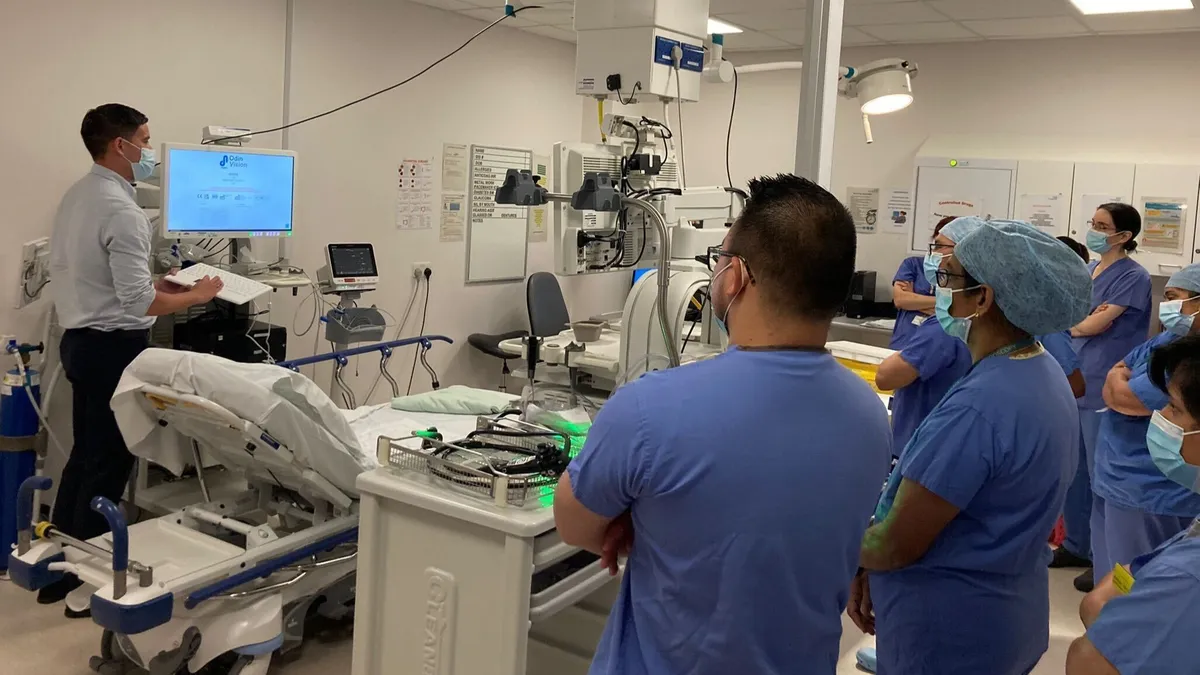
Olympus agrees to $80M takeover of artificial intelligence endoscopy startup Odin Vision
The acquisition gives Olympus control of cloud-based AI systems to help detect and characterize cancerous and precancerous tissues during colonoscopies and gastroscopies.
By Nick Paul Taylor • Dec. 21, 2022 -
As diabetes technology advances, blood glucose control declines: study
Mexican-American and non-Hispanic Black adults saw the greatest drop as the proportion of patients with glycemic control fell from 29.2% between 1988 and 1994 to 27.5% between 2013 and 2020.
By Nick Paul Taylor • Dec. 21, 2022 -
Medtronic starts US robotic surgery trial in push to challenge Intuitive
Medtronic delayed the Hugo program to make changes in light of surgeon feedback, but now plans to evaluate the use of the surgical robot in urologic procedures, facing off against competitor Intuitive.
By Nick Paul Taylor • Dec. 20, 2022 -
MDR costs are driving manufacturers to pull products from European market: report
The report adds to evidence that makers of products used for pediatric patients and rare diseases have been particularly hard hit.
By Nick Paul Taylor • Dec. 20, 2022 -
Fraud risk increased among Medicare DME suppliers who enrolled under pandemic waivers: GAO
Of 208 providers that had their Medicare enrollment revoked between 2020 and 2022, 83% were durable medical equipment suppliers, according to a report from the Government Accountability Office.
By Elise Reuter • Dec. 19, 2022 -
J&J subsidiaries accuse former Auris employees of stealing robotic surgery trade secrets
The lawsuit claims former Auris employees engaged in “shameless, systematic and ongoing misappropriation of trade secrets.”
By Nick Paul Taylor • Dec. 19, 2022 -
Click wins FDA breakthrough designation for prescription digital migraine therapy
The designation would give Click Therapeutics expedited review of its preventive treatment for episodic migraine in adults.
By Nick Paul Taylor • Dec. 19, 2022 -
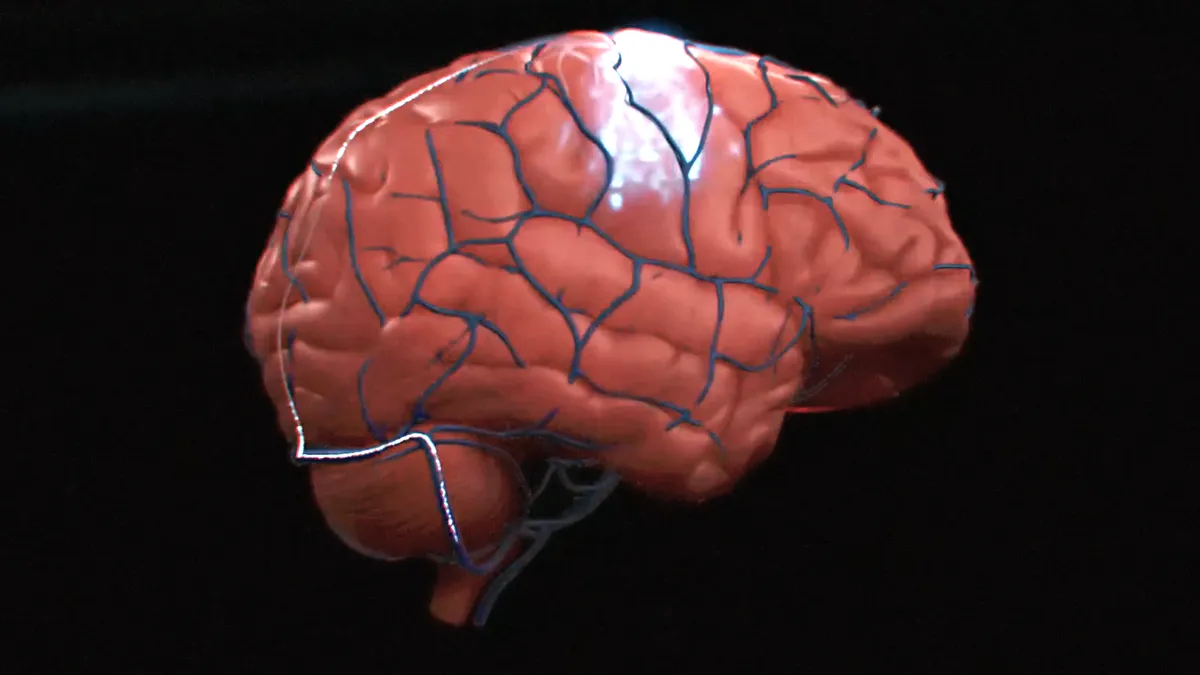
Gates, Bezos invest in Synchron’s brain computer interface
A $75 million investment will accelerate the development of a drill-free implant to help patients with upper-limb paralysis.
By Peter Green • Dec. 16, 2022 -
Siemens, GE Healthcare are interested in Medtronic spinoff units: Bloomberg
Medtronic said in October it plans to divest its patient monitoring and respiratory interventions businesses.
By Elise Reuter • Dec. 15, 2022 -
Masimo links passive health monitors to home stereos amid legal row with Apple
The move integrates the consumer audio brands Masimo bought in April with its wearable health monitors.
By Peter Green • Dec. 15, 2022 -
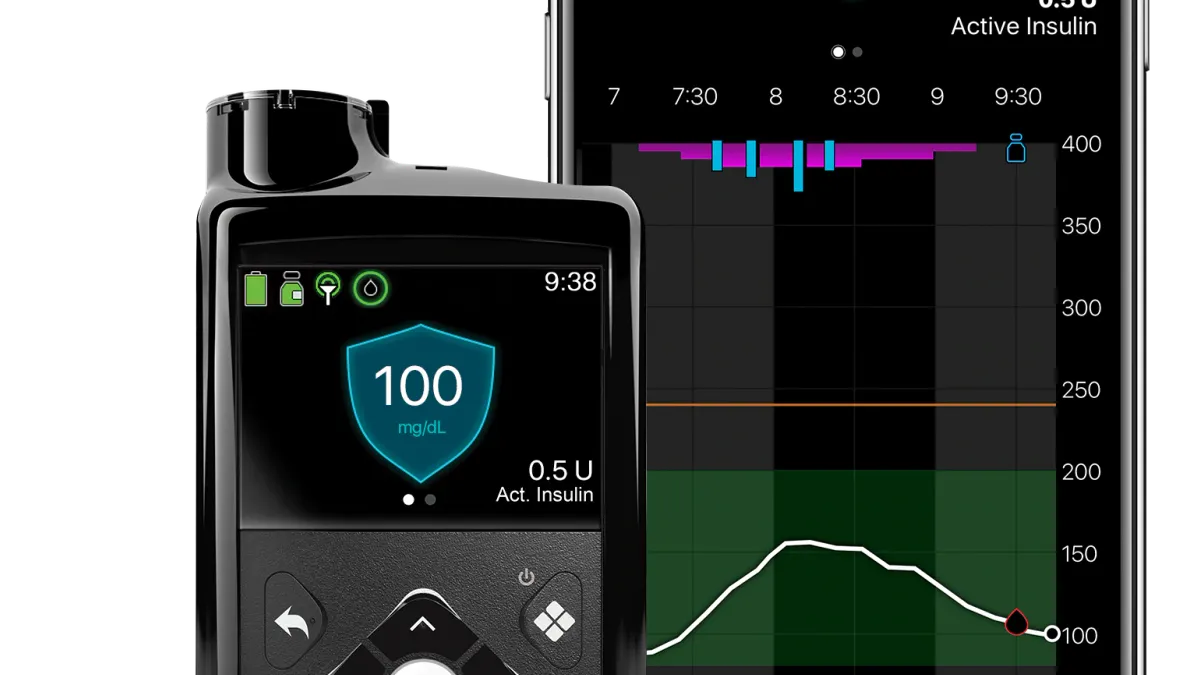
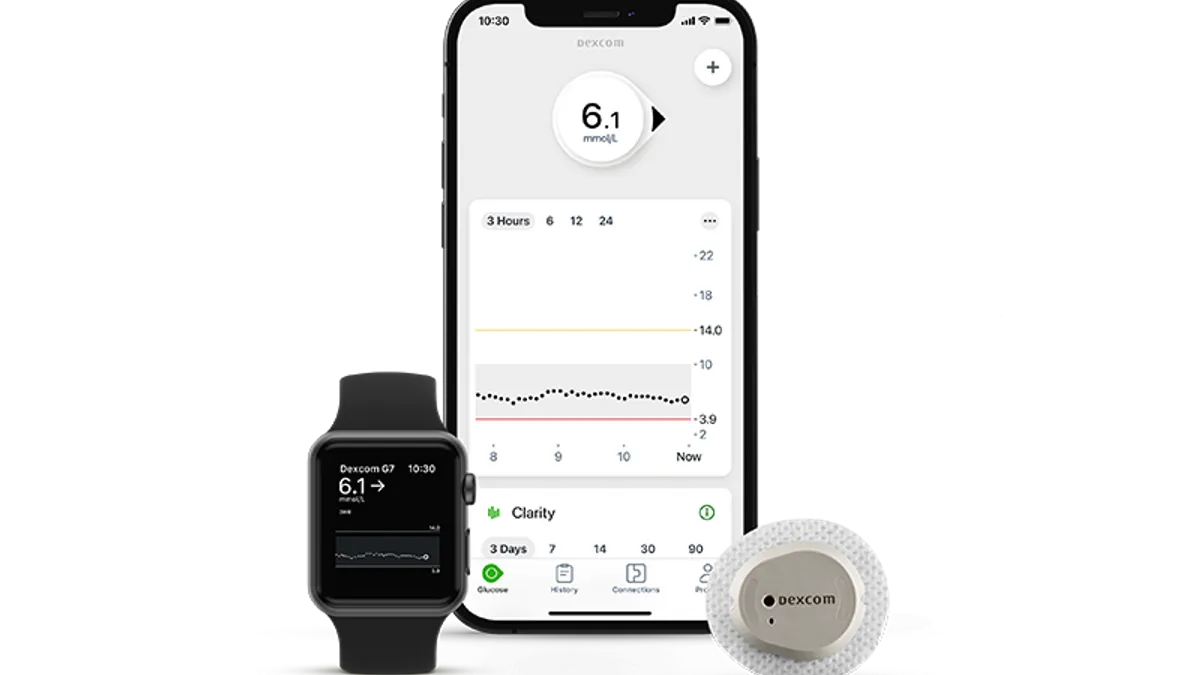
Dexcom’s Jake Leach discusses preparations for G7 launch next year
The diabetes tech company is in conversations with payers and is opening a new manufacturing plant ahead of the planned launch of its newest CGM, the COO said.
By Elise Reuter • Dec. 15, 2022 -
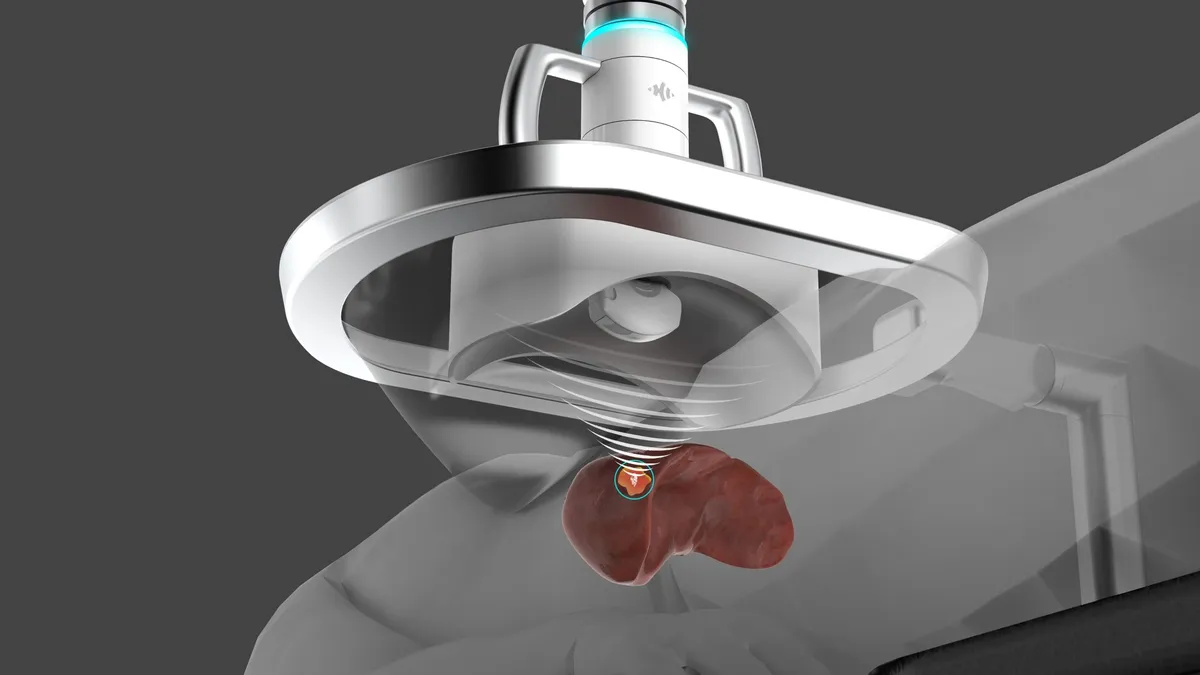
J&J leads $85M investment in HistoSonics ahead of liver cancer device authorization
HistoSonics is developing a device that delivers focused ultrasound to destroy and liquefy unwanted tissue and tumors without the need for surgical incisions.
By Nick Paul Taylor • Dec. 14, 2022 -
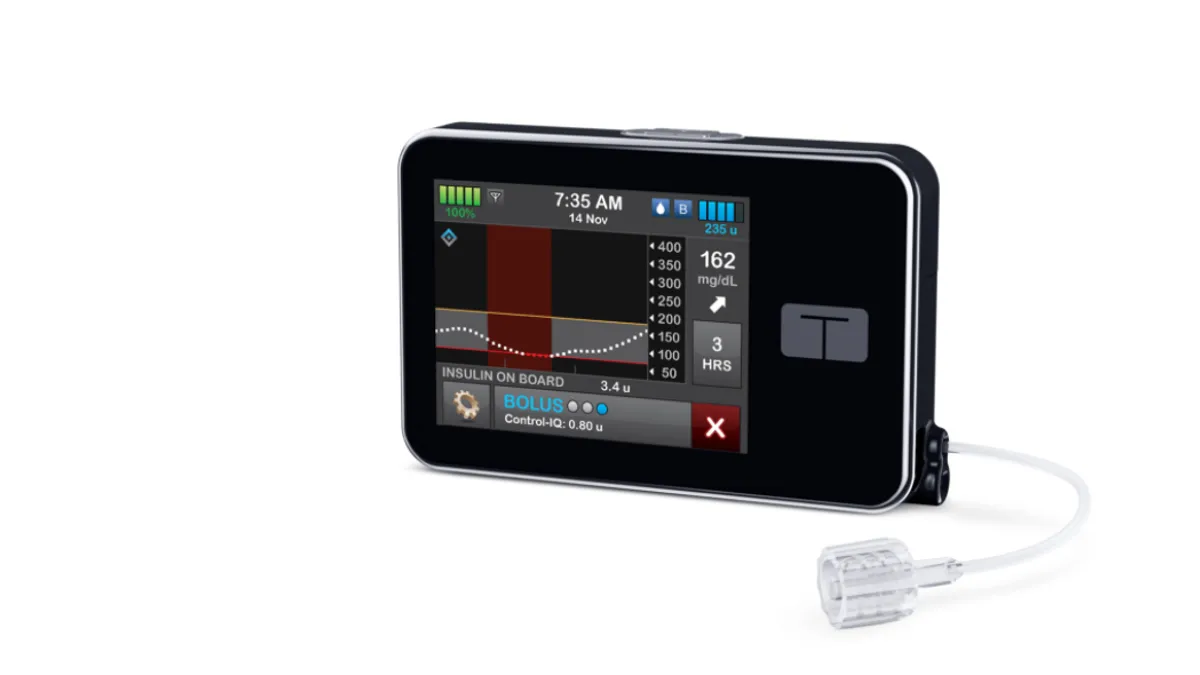
Tandem agrees to buy AMF, challenging Insulet for insulin pump patch market
The deal will give Tandem control of Sigi, a reusable, rechargeable patch pump that is designed to be smaller and lighter than existing devices.
By Nick Paul Taylor • Dec. 14, 2022