Medical Devices: Page 85
-
GE advances healthcare spinoff plan, providing close look at workings of the unit
Localization requirements, third-party servicers, and more sophisticated data security threats are cited as risks to the healthcare unit.
By Nick Paul Taylor • Oct. 13, 2022 -
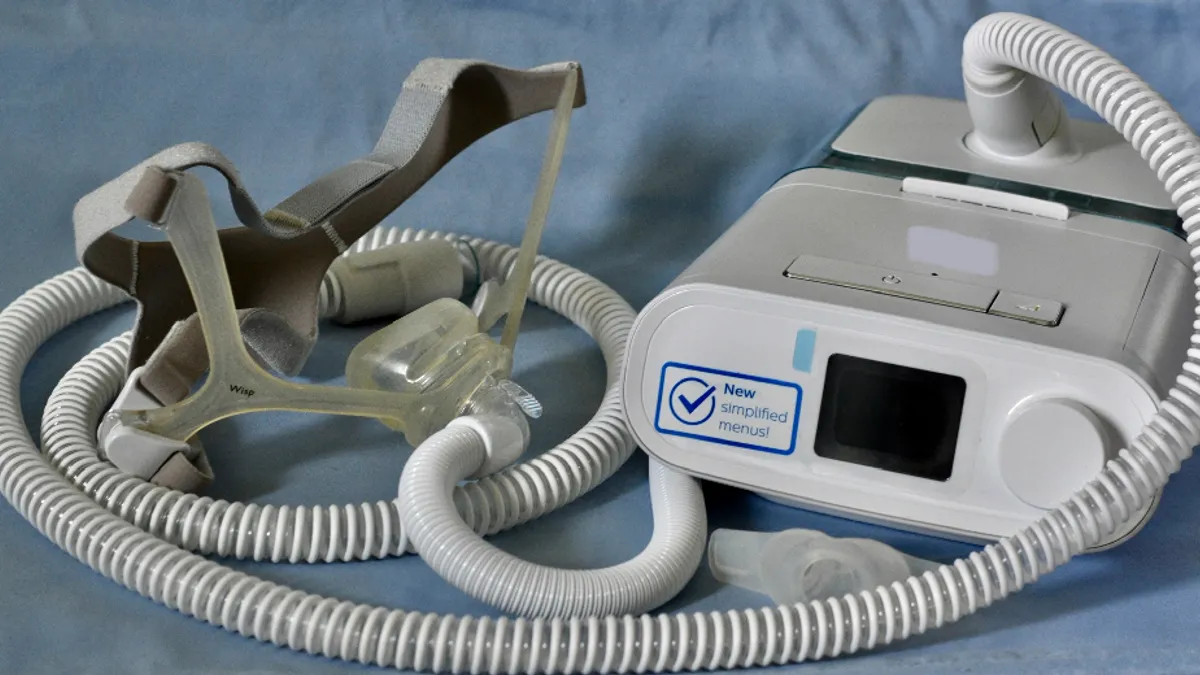
Philips says Q3 revenue fell, records $1.26B charge for Respironics; shares plunge
The Dutch-based medtech company remains dogged by sleep apnea machine recalls and supply chain issues.
By Peter Green • Oct. 12, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA starts advisory program pilot to reduce ‘valley of death’ risk for medical devices
A plan to ensure more medical devices pass from testing to clinical application aims to help dozens of devices through the approval process each year.
By Nick Paul Taylor • Oct. 12, 2022 -
‘Do-it-yourself’ artificial pancreas system beats production-line pump in controlled trial
Type 1 diabetes patients who used a customizable, open-source artificial pancreas, an insulin pump and a Dexcom G6 CGM found it more effective at controlling blood glucose than conventional sensor-augmented insulin pumps.
By Nick Paul Taylor • Oct. 12, 2022 -
FDA finalizes postapproval study guidance in light of AdvaMed, Foundation Medicine feedback
The agency resisted calls to give sponsors more time to prepare protocols for post-approval studies.
By Nick Paul Taylor • Oct. 11, 2022 -
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Abbott’s FreeStyle Libre 2 CGM beats fingerstick testing in independent clinical trial
Going into the study, the researchers were unsure if CGMs with optional alarms for high and low blood glucose levels benefit patients with Type 1 diabetes.
By Nick Paul Taylor • Oct. 11, 2022 -
NovaSight’s digital treatment for lazy eye gets FDA nod, providing alternative to patching
A randomized controlled trial found the digital device to be as effective as wearing an eye patch, the current gold standard for treatment, opening the way for more children to complete necessary therapy for amblyopia.
By Nick Paul Taylor • Oct. 10, 2022 -
Owlet seeks clearance of blood-oxygen-measuring baby sock after FDA warning letter
Owlet now is a step closer to selling a prescription medical device designed to alert parents when their baby’s heart rate or oxygen saturation levels move outside of prescribed ranges.
By Nick Paul Taylor • Oct. 10, 2022 -
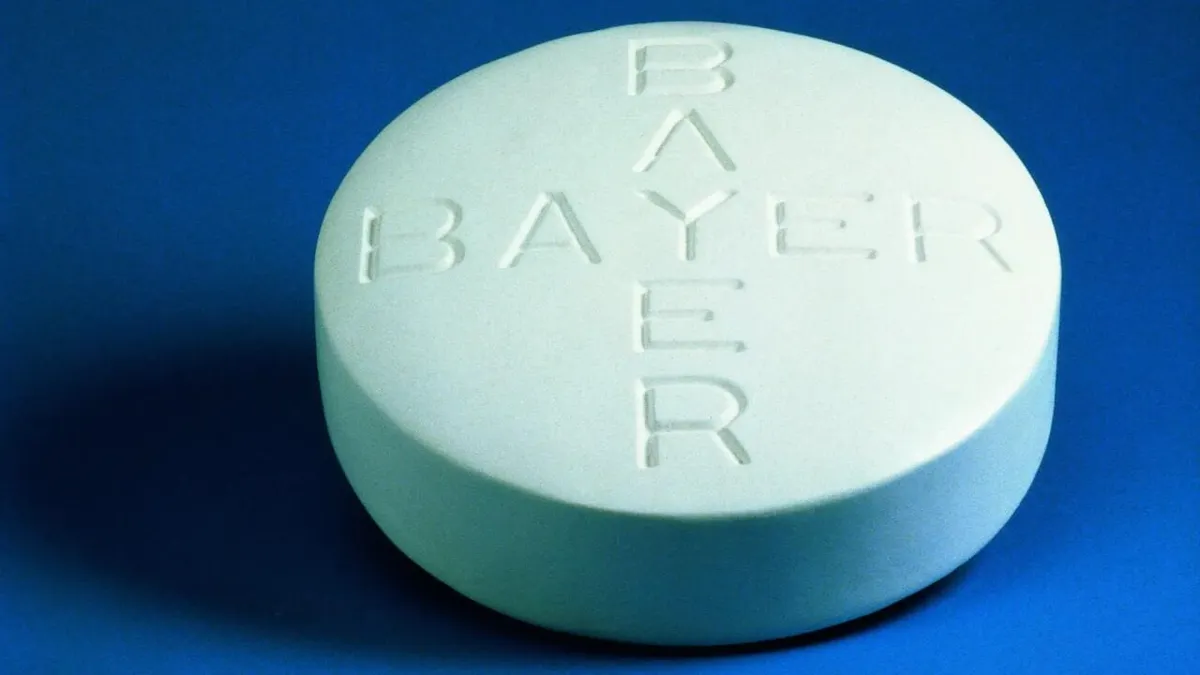
‘Inadequate’ progress of Bayer’s study on birth-control implant called out by FDA as patients drop out
The FDA saw a rise in patients dropping out of a study on the Essure birth-control implant, prompting it to tell Bayer to develop and implement strategies to ensure the work continues.
By Nick Paul Taylor • Oct. 7, 2022 -

 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Dexcom, Abbott have ‘massive opportunity’ with new CGM coverage proposal: analysts
Analysts at J.P. Morgan said the proposal reads “very favorably” for the two leading makers of continuous glucose monitors and unlocks “a major near-term driver for growth.”
By Nick Paul Taylor • Oct. 7, 2022 -
BD discloses cybersecurity vulnerability in cervical cytology processing machine
The company is advising users to address the threat by restricting access to the instrument while it works on a software patch.
By Nick Paul Taylor • Oct. 6, 2022 -
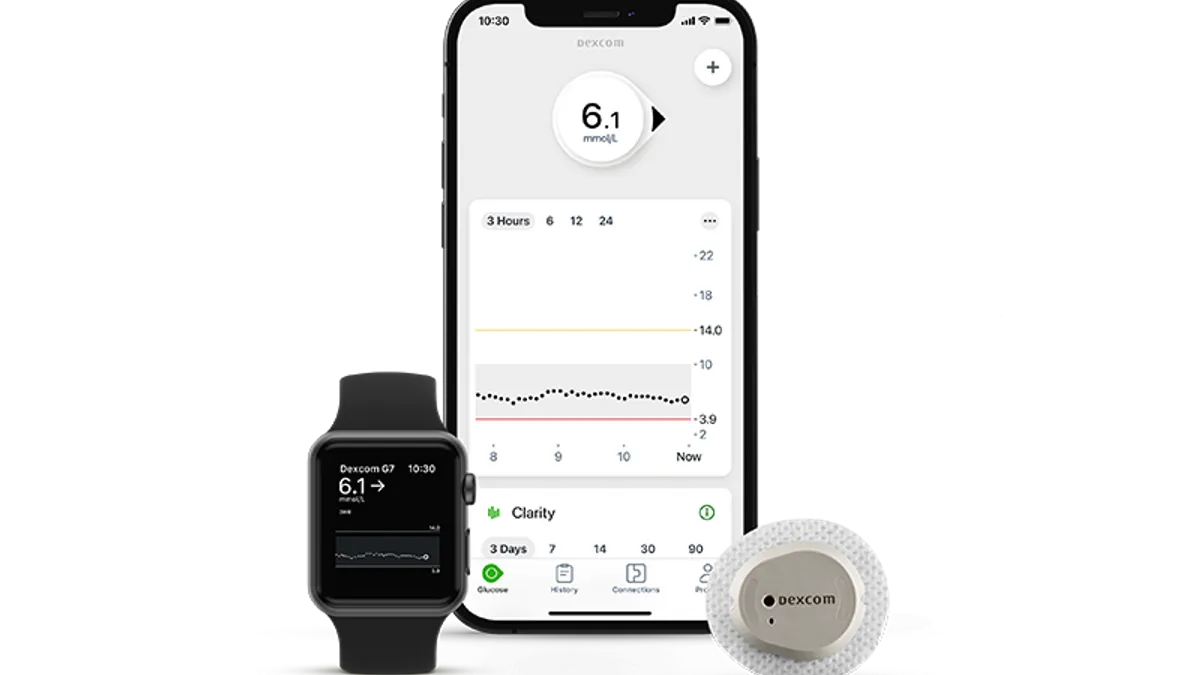
Dexcom starts global rollout of G7 CGM system, launching device in U.K. and Germany
Software updates have delayed the introduction of the product in the U.S. at a time when the company seeks to take market share from rival Abbott.
By Nick Paul Taylor • Oct. 6, 2022 -
Deep Dive
Medtech companies shift strategy as more orthopedic procedures move to ambulatory surgical centers
Many ASCs are looking for cashless options to get capital equipment as they offset the cost of new buildings, Zimmer Biomet COO Ivan Tornos said.
By Elise Reuter • Oct. 5, 2022 -
Medical machine-learning studies lack high-quality clinical trials, review shows
The findings “highlight areas of concern regarding the quality of medical machine learning RCTs and suggest opportunities to improve reporting.”
By Nick Paul Taylor • Oct. 4, 2022 -
LivaNova recall of open-heart-surgery blood pump designated as Class I event by FDA
A software malfunction can mistakenly freeze the machine, set off an alarm that can’t be muted and lead to the heart pump stopping during surgery.
By Nick Paul Taylor • Updated Oct. 12, 2022 -
Medtech groups praise MDUFA passage as legislators plan to include reforms in end-of-year bill
The legislation will provide the FDA with as much as $1.9 billion over the next five years, but changes to diagnostics and medical device cybersecurity were slashed from a "clean version" of the bill.
By Elise Reuter • Oct. 3, 2022 -
Illumina ushers in $200 genome with the launch of new sequencers
The company’s machines are part of a long line of products that have enabled Illumina to dominate the market for short-read sequencing.
By Nick Paul Taylor • Oct. 3, 2022 -
European AI Act could have ‘significant impact’ on manufacturers, medtech group warns
“Overregulation and misalignment” could create uncertainty and stop products from coming to market, MedTech Europe says.
By Nick Paul Taylor • Oct. 3, 2022 -
Pulse oximeter bias delayed treatment of Black COVID-19 patients by hours: study
An author of the paper called for manufacturers to “go back to the drawing board to provide clinicians with a tool that is free from bias.”
By Nick Paul Taylor • Sept. 30, 2022 -
Philips
Philips shareholders officially appoint Jakobs as CEO, president
Jakobs is Philips’ chief business leader for the connected care unit and has helped with the company’s recall of millions of sleep apnea and ventilator devices.
By Ricky Zipp • Sept. 30, 2022 -
Q&A
Friday Q&A: iRhythm CEO Quentin Blackford on how the heart monitor maker plans to reach $1B in sales
Continuing the company’s success in the U.S. and expanding internationally are the two biggest factors to achieving its $1 billion forecast, says Blackford.
By Ricky Zipp • Sept. 30, 2022 -
Veterans Affairs, FDA partner to accelerate medical device development
The goal is to provide standardized, off-the-shelf tests that can streamline the regulatory process and reduce time to market.
By Nick Paul Taylor • Sept. 30, 2022 -
Congress passes continuing resolution reauthorizing FDA user fees; bill heads to Biden for signature
President Biden is expected to sign last-minute deal to fund the government, that includes a “clean” bill without amendments to fund FDA review activities for five years.
By Elise Reuter • Updated Sept. 30, 2022 -
Bionic pancreas led to lower blood sugar levels than standard of care: study
The results point to the potential for a simplified approach for automated insulin delivery that could be particularly useful for patients without regular access to an endocrinologist.
By Elise Reuter • Sept. 29, 2022 -
Cyber risks identified in Canon Medical product used to view medical images, security firm says
Vulnerabilities in Canon Medical’s Vitrea View product could provide access to patient information and other services, according to cybersecurity firm Trustwave Spiderlab.
By Ricky Zipp • Updated Sept. 30, 2022