Medical Devices: Page 84
-
Accelerate Diagnostics withdraws BD-partnered device after FDA demands 510(k) clearance
An analyst said they were “somewhat surprised” by the FDA’s decision as “Arc does not directly lead to a patient result.”
By Nick Paul Taylor • Oct. 25, 2022 -
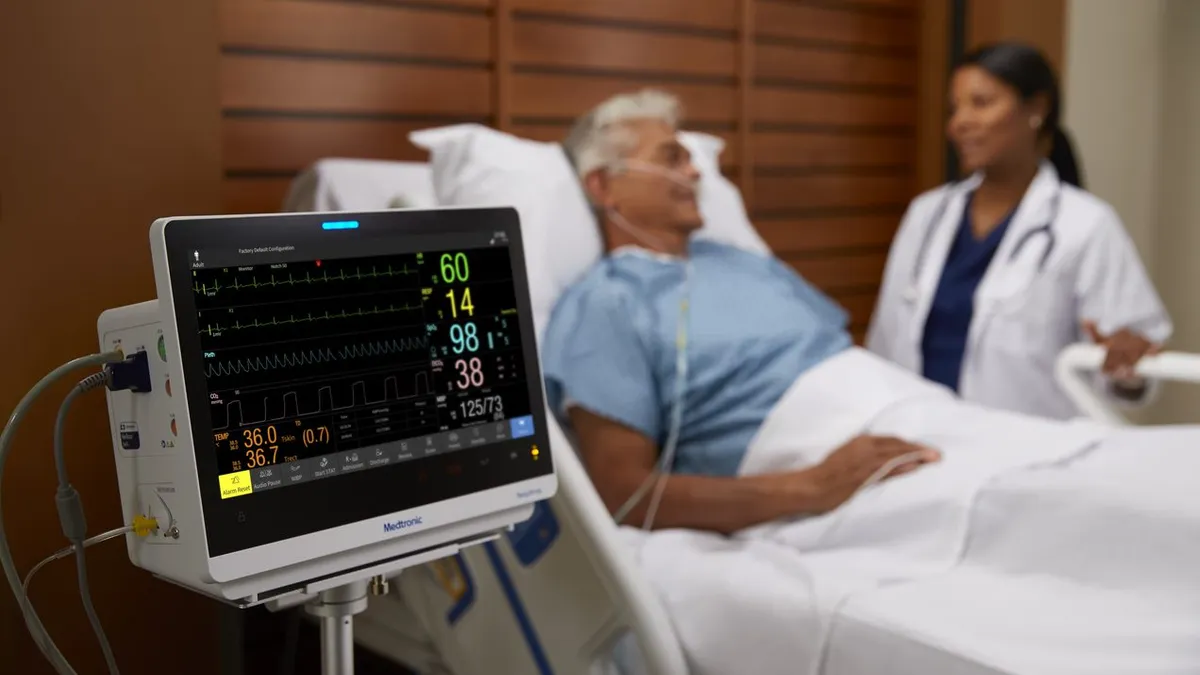
Medtronic’s planned spinoff ‘doesn’t move the needle’ in growth strategy: analysts
Combining patient monitoring and respiratory intervention businesses into a separate company may not be enough to address investor concerns.
By Elise Reuter • Oct. 24, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Medtronic will spin out slower-growth patient monitoring, respiratory units to focus on core businesses
The segments will form a new connected care company, allowing Medtronic to invest more resources into faster-growing areas.
By Elise Reuter • Oct. 24, 2022 -
Philips to cut 4,000 jobs amid net loss related to recalls, declining sales
The layoffs will cost Philips $300 million over the coming quarters and save it about the same amount annually thereafter.
By Nick Paul Taylor • Oct. 24, 2022 -
Boston Scientific, Edwards set to report Q3 results after ‘encouraging start’ to medtech earnings season
The companies may post results that show procedure volumes are recovering, analysts say.
By Nick Paul Taylor • Oct. 24, 2022 -
New Philips CEO Jakobs apologizes for handling of sleep apnea device recall
In a blog post, the executive says he’s “deeply sorry” for chaotic recall of faulty respirators.
By Peter Green • Oct. 21, 2022 -
Abiomed wraps up Impella post-approval studies requested by FDA
Abiomed’s studies linked the device to a 22% to 45% improvement in the volume of blood pumped after 90 days, suggesting it’s a viable alternative to balloon therapy.
By Nick Paul Taylor • Oct. 21, 2022 -
FDA moves to make effect on health equity part of breakthrough device considerations
If it finalizes the proposal, the agency will expand its breakthrough program to devices that improve accessibility.
By Nick Paul Taylor • Oct. 21, 2022 -
Abbott Q3 device sales slowed by supply chain pressures; COVID-19 tests exceed expectations
The company raised its earnings forecast for 2022 on the back of an expected $7.8 billion in annual sales related to COVID-19 testing.
By Elise Reuter • Oct. 19, 2022 -
BD forms co-promotion pact with Magnolia to reduce blood culture contamination
Magnolia’s blood collection device diverts the first 1.5 to 2 milliliters of blood samples that may be contaminated with flecks of skin, paving the way for more accurate diagnosis of sepsis.
By Nick Paul Taylor • Oct. 19, 2022 -
FDA seeks feedback on plans to draft and finalize medical device guidance in 2023
The agency’s review includes guidance on transitioning away from emergency use authorizations, while abandoning plans for guidance on software as a medical device.
By Nick Paul Taylor • Oct. 19, 2022 -
Cordis to spend $235M to buy Swiss maker of alternative to paclitaxel balloons
As regulatory and sales milestones are hit, Cordis could pay up to $900 million in total.
By Nick Paul Taylor • Oct. 19, 2022 -
Intuitive’s robotic surgery volumes recovered in Q3; shares climb
As hospital staffing increased and COVID-19 wariness eased, the number of procedures performed with the company’s hallmark da Vinci system rose 20% in the third quarter from a year earlier.
By Peter Green • Oct. 18, 2022 -
J&J touts rising procedure volumes, lowers full-year forecast on foreign-exchange concerns
Analysts said the results were an “encouraging start” to medtech earnings for the third quarter.
By Elise Reuter • Oct. 18, 2022 -
Lucid enters OTC hearing aid market, undercutting rivals with devices starting at $200
Lucid joins Sony and the Bose-partnered Lexie Hearing with aids priced thousands of dollars below prescription devices, putting auditory assistance within reach of tens of millions of Americans.
By Nick Paul Taylor • Oct. 18, 2022 -
Coloplast told by FDA to keep transvaginal mesh off market after 36-month surveillance study
The mesh provides similar effectiveness and safety outcomes to native tissue repair but also comes with additional risks, regulators concluded.
By Nick Paul Taylor • Oct. 18, 2022 -
Medtronic, in a first, secures expanded label for cardiac pacing lead
The approval is part of Medtronic’s push to expand its share of the market for cardiac conduction system pacing.
By Nick Paul Taylor • Oct. 18, 2022 -
Insulet flags battery problem with Omnipod DASH system
The company is expected to spend $35 million to $45 million replacing all of its personal diabetes manager devices after reports that batteries in the handheld controller may be swelling, leaking fluid or overheating.
By Elise Reuter • Oct. 17, 2022 -

 Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
Courtesy of https://news.medtronic.com/Left-Ventricular-Assist-Device-For-Advanced-Heart-Failure#assets_34137_10-122:19299
Medtronic’s faulty HVAD controllers get an emergency-only software fix
The new controllers will be available to all healthcare providers with a patient on HVAD support, but the company cautioned that the software should only be used after previous attempts to restart the pump have failed.
By Elise Reuter • Oct. 17, 2022 -
Johnson & Johnson, Alcon agree on $75M settlement to resolve contact-lens antitrust claims
A class-action suit accused the manufacturers of violating federal antitrust law by setting the same minimum price for their lenses.
By Nick Paul Taylor • Oct. 17, 2022 -
Sponsored by Esper
Why Android is the future of connected HealthTech devices
How Android can make your connected HealthTech device strategy a success.
Oct. 17, 2022 -
 Retrieved from Abbott/PRNewswire on June 15, 2020
Retrieved from Abbott/PRNewswire on June 15, 2020
Abbott, J&J and Intuitive to lead off medtech earnings season; procedures, supply chain in spotlight
Results from the three companies are expected to shed light on how the economic slowdown is affecting procedure trends in the industry.
By Nick Paul Taylor • Oct. 17, 2022 -
Hospital staff shortages spurred drop in TAVR procedures in Q3, surgeon survey shows
Staffing at hospitals is expected to remain disrupted until at least 2024, reducing the number of surgeries, more than 40% of respondents said.
By Nick Paul Taylor • Oct. 14, 2022 -
Bose-partnered Lexie to launch $999 OTC hearing aid, challenging Sony, Eargo in nascent market
Lexie has matched the price of the first Sony device and is seeking to differentiate itself through the use of a rechargeable battery.
By Nick Paul Taylor • Oct. 14, 2022 -
Sony begins sale of OTC hearing aids with entry-level price of $999
Lower-cost, over-the-counter hearing aids are becoming available to the 70% of older Americans who need the devices but don’t have them.
By Peter Green • Oct. 13, 2022