Medical Devices: Page 86
-
B. Braun acquires suite of catheter securement products from Starboard medical for undisclosed amount
The six acquired devices would improve patient care and extend B. Braun’s position in IV therapy, the company says.
By Elise Reuter • Sept. 28, 2022 -
FDA makes case for ‘new regulatory paradigm’ amid hurdles in software-oversight program
The agency seeks to tailor requirements based on the latest science, the benefits and risks posed by devices, their real-world performance and their contribution to promoting health equity.
By Nick Paul Taylor • Sept. 27, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Hospital procedures have remained steady since H2 in boost for medtechs, survey finds
Evercore ISI analysts said the findings bode well for device companies, even as hospitals expect capital spending to drop next year.
By Elise Reuter • Sept. 26, 2022 -
Philips’ latest BiPAP machine recall labeled Class I event by FDA
Certain bi-level positive airway pressure devices feature plastic that could cause machines to stop working suddenly, potentially leading to serious injury or death, the FDA said Friday.
By Nick Paul Taylor • Sept. 26, 2022 -
Sponsored by Esper
Pros and cons of custom devices for HealthTech use cases
To meet the needs of both your specific use case and strict regulations, should you go custom?
Sept. 26, 2022 -
Device makers with ethylene oxide facilities at risk of lawsuits after Sterigenics loss: Needham
The $363 million award in the Sterigenics lawsuit “could lead to increased aggressiveness on the part of attorneys,” Needham analysts warned in a Friday note to investors.
By Ricky Zipp • Sept. 23, 2022 -
Forget oximeters, smartphone cameras detect 79% of cases of low blood oxygen in small study
If the results are validated by wider studies, physicians will have a new tool to increase the number of people who can be monitored for chronic and acute respiratory conditions.
By Nick Paul Taylor • Sept. 23, 2022 -
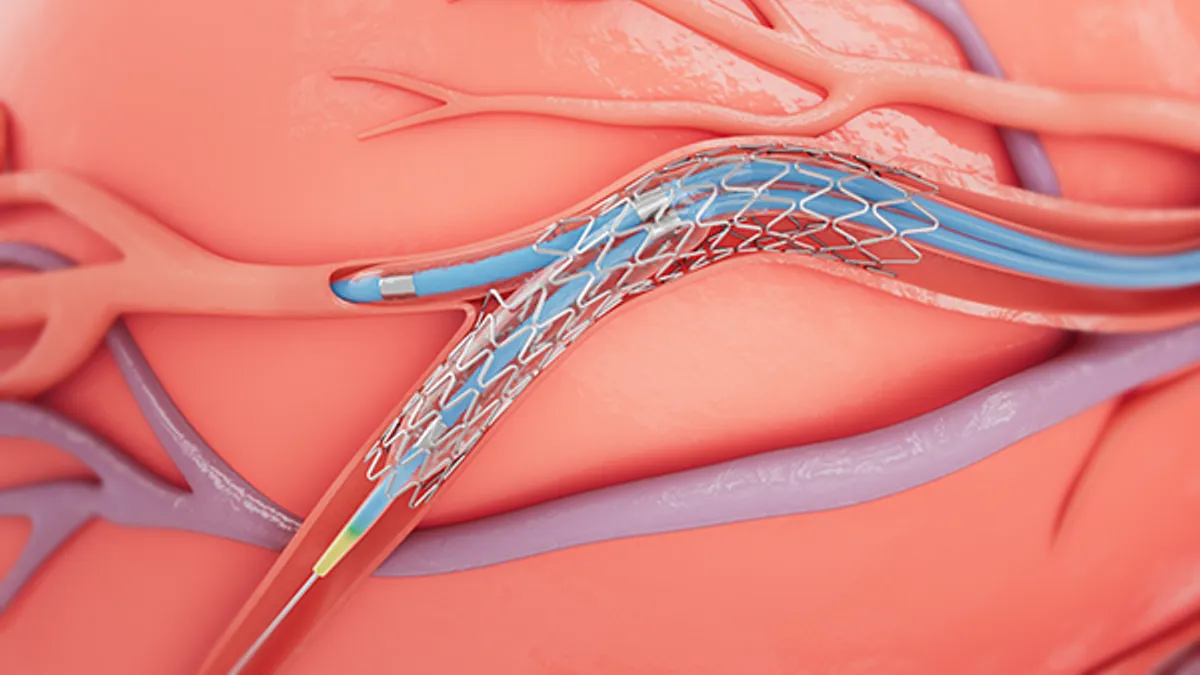
 Courtesy of https://filecache.mediaroom.com/mr5mr_medtronic/183509/SURTAVI%20%28002%29%209.21%20600pix.png
Courtesy of https://filecache.mediaroom.com/mr5mr_medtronic/183509/SURTAVI%20%28002%29%209.21%20600pix.png
Medtronic gets first FDA approval for drug-eluting stent in bifurcation lesions
Researchers have found the one-device, provisional stenting method delivers better results than two stents in some patients.
By Nick Paul Taylor • Sept. 22, 2022 -

 Courtesy of https://www.dbl-diabetes.com/wp-content/uploads/2022/03/1114x708-dblg1-system-overview-EN.jpg.webp
Courtesy of https://www.dbl-diabetes.com/wp-content/uploads/2022/03/1114x708-dblg1-system-overview-EN.jpg.webp
Diabeloop’s insulin algorithm shows 17-point rise in time-in-range from year-long patient study
About half of the people using its device achieve the recommended outcomes, versus 27% of those using standard treatment.
By Nick Paul Taylor • Sept. 22, 2022 -
Insulet’s Omnipod 5 gains CE Mark for European release
After months of regulatory delays, Insulet’s newest insulin pump can now be marketed in the U.S. and European Union.
By Nick Paul Taylor • Sept. 21, 2022 -
Abbott’s FreeStyle Libre linked to 67% drop in hospitalizations for Type 2 diabetes patients: study
After switching to the continuous glucose monitor, patients had fewer acute diabetes-related events such as severe hypoglycemia and diabetic ketoacidosis.
By Nick Paul Taylor • Sept. 21, 2022 -
Medtronic issues urgent notice about MiniMed insulin pump system vulnerability
Unauthorized users could hack into the device while it’s being set up, and cause the pump to deliver too much or too little insulin, potentially resulting in outcomes including death.
By Nick Paul Taylor • Sept. 21, 2022 -
J&J opens new research hub near San Francisco
The roughly 200,000-square-foot facility will house about 400 employees and more than double the size of the drugmaker’s presence in San Francisco.
By Delilah Alvarado • Sept. 20, 2022 -
Zimmer Biomet gets FDA clearance for Identity shoulder system
Ivan Tornos, the company’s chief operating officer, said in August that it would be Zimmer’s biggest shoulder launch in five years.
By Elise Reuter • Sept. 20, 2022 -
Jury decides against Sterigenics in ethylene oxide trial, awarding $363M to the sole plaintiff
Sterigenics has more than 760 lawsuits pending against it in the local court for toxic emissions previously emitted by the plant.
By Nick Paul Taylor • Sept. 20, 2022 -
Hurricane Fiona unlikely to significantly disrupt medical device supply: analysts
Medtech management teams expect fewer problems than after Hurricane Maria in 2017, noting that Hurricane Fiona is less severe and companies are better prepared.
By Nick Paul Taylor • Sept. 20, 2022 -
Abbott’s latest Amulet-Watchman data show more mixed results for the heart devices
The latest head-to-head trial adds to the growing rivalry between Abbott and Boston Scientific in the left atrial appendage closure space.
By Ricky Zipp • Sept. 19, 2022 -
FDA updates advice about Medtronic endotracheal tubes after Class I recall, patient death reports
The FDA provided more detailed recommendations on preventing and responding to airway obstruction that may occur when using some Medtronic endotracheal tubes.
By Peter Green • Sept. 19, 2022 -
Medtronic starts full US commercial release of updated Evolut TAVR system
Medtronic expects the device’s new features to increase its share of the multibillion-dollar heart valve replacement market.
By Nick Paul Taylor • Sept. 19, 2022 -
Edwards gets FDA nod for mitral regurgitation treatment
The approval would bring a U.S. competitor to rival Abbott’s MitraClip device.
By Elise Reuter • Sept. 16, 2022 -
Racial bias in pulse oximeters to be subject of FDA panel
The design of the ubiquitous fingertip monitors has inadvertently caused false blood oxygen readings in darker-skinned patients, a problem the FDA hopes to fix.
By Peter Green • Sept. 16, 2022 -
Q&A
Edwards head of TAVR, Larry Wood, says more urgency needed in treating heart valve disease
Continued staffing shortages at hospitals are delaying the non-invasive aortic valve procedures for patients in need.
By Elise Reuter • Sept. 16, 2022 -
Baxter’s recall of solution sets used in chemotherapy labeled Class I by FDA amid complaints of leaks
The regulator says the leaks could kill, but with 83 complaints it has not received reports of patient injuries or deaths.
By Ricky Zipp • Sept. 15, 2022 -
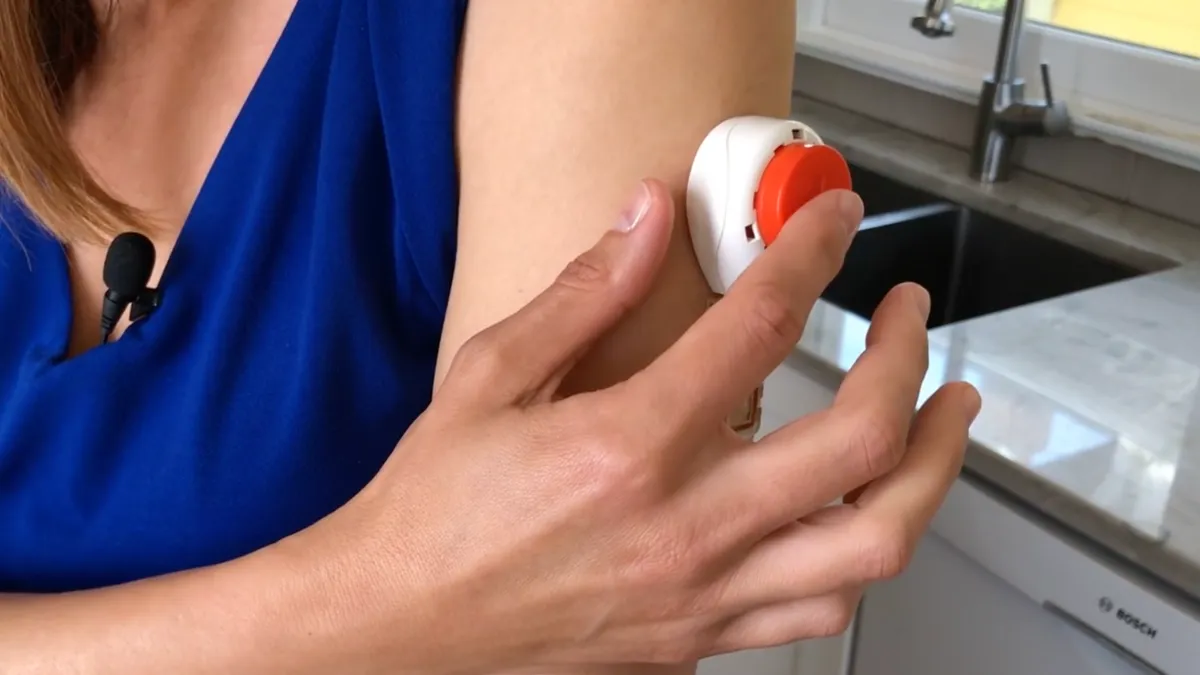
 Retrieved from https://www.tassoinc.com/product-use-videos on September 15, 2022
Retrieved from https://www.tassoinc.com/product-use-videos on September 15, 2022
Tasso’s at-home blood collection lancet wins FDA clearance
Tasso is pitching the device as an enabler of decentralized clinical trials because it could simplify the collection of blood at home.
By Nick Paul Taylor • Sept. 15, 2022 -
FBI warns of cyber risks from legacy medical devices
Outdated device software can put data, patient safety and hospitals’ operations at risk, the top U.S. law enforcement agency warned.
By Ricky Zipp • Sept. 15, 2022