Medical Devices: Page 87
-
Medtech firms increasingly positive about future as staffing, inflation pressures ease, say RBC analysts
Hospital staffing and supply chain issues are expected to improve, according to comments from Boston Scientific, J&J, Medtronic, and others.
By Nick Paul Taylor • Sept. 15, 2022 -
Medtronic reports two deaths related to endotracheal tube recall
The company also received reports of three injuries related to airway obstruction with the tubes, adding to the company’s recent recall woes.
By Elise Reuter • Updated Sept. 14, 2022 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
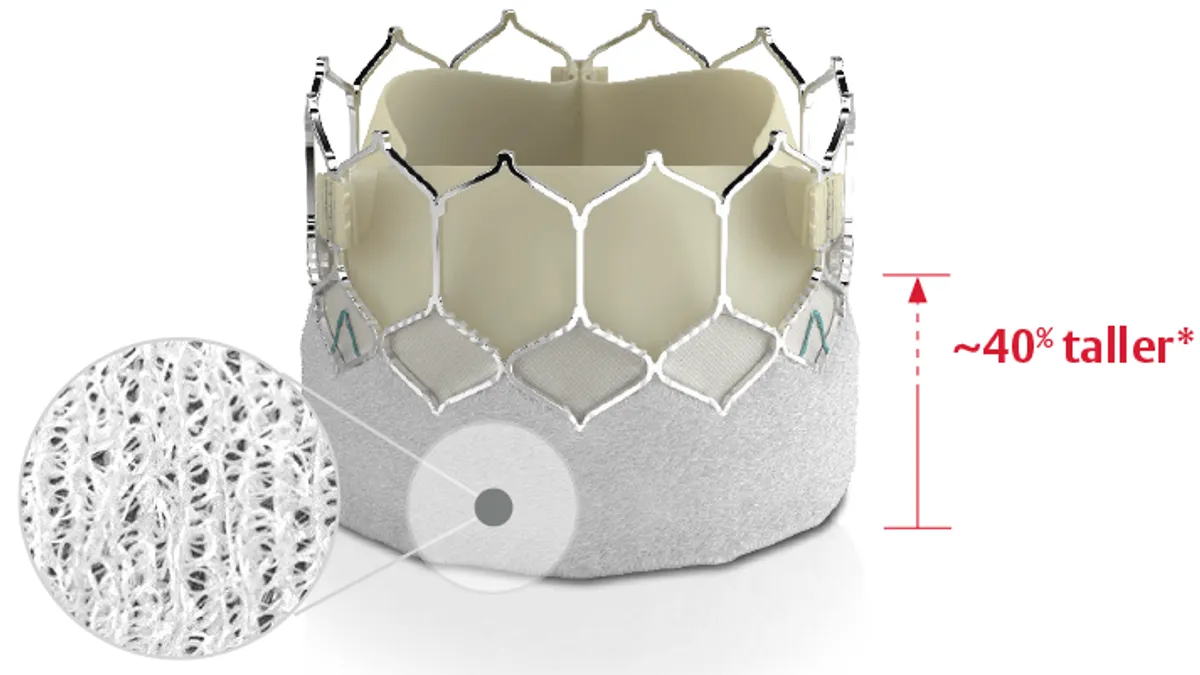
Edwards updates Sapien TAVR device for greater durability
The device uses Edwards’ Resilia tissue technology to enable dry storage and potentially extend device durability.
By Nick Paul Taylor • Sept. 14, 2022 -
Sony partners with hearing aid specialist to enter new over-the-counter market
Sony has identified the U.S. regulatory change as a chance to use its brand and audio technology to add a new growth driver to its portfolio.
By Nick Paul Taylor • Sept. 14, 2022 -
Investor lawsuit accuses Medtronic of failing to disclose insulin pump problems
The plaintiffs claim Medtronic failed to disclose that its product quality control systems were inadequate and that it was noncompliant with regulations.
By Nick Paul Taylor • Sept. 13, 2022 -
J&J agrees to settle class action pelvic mesh suits in Australia for more than $200M
Shine Lawyers, the firm representing the patients, said that the Federal Court of Australia still needs to approve the terms of the agreement.
By Ricky Zipp • Sept. 13, 2022 -
 Retrieved from Catheter Precision website on September 13, 2022
Retrieved from Catheter Precision website on September 13, 2022
Catheter Precision agrees to reverse merger, securing cash to target cardiac electrophysiology market
By merging into Ra Medical Systems, Catheter Precision has access to public markets and a pool of cash needed to bring its product to market.
By Nick Paul Taylor • Sept. 13, 2022 -

 Retrieved from Titan on September 12, 2022
Retrieved from Titan on September 12, 2022
Medtronic signs development deal with surgical robot-maker Titan Medical
Medtronic placed a $2.6 million order for some of the company’s instruments and cameras, and its interest in Titan should help the smaller firm as it seeks to win approval for its device and market it globally.
By Elise Reuter • Sept. 12, 2022 -
Philips says French prosecutors investigating respirator recall
While the company confirmed it faces lawsuits in France, Philips said it’s too early “to speculate about any potential exposure.”
By Elise Reuter • Sept. 9, 2022 -
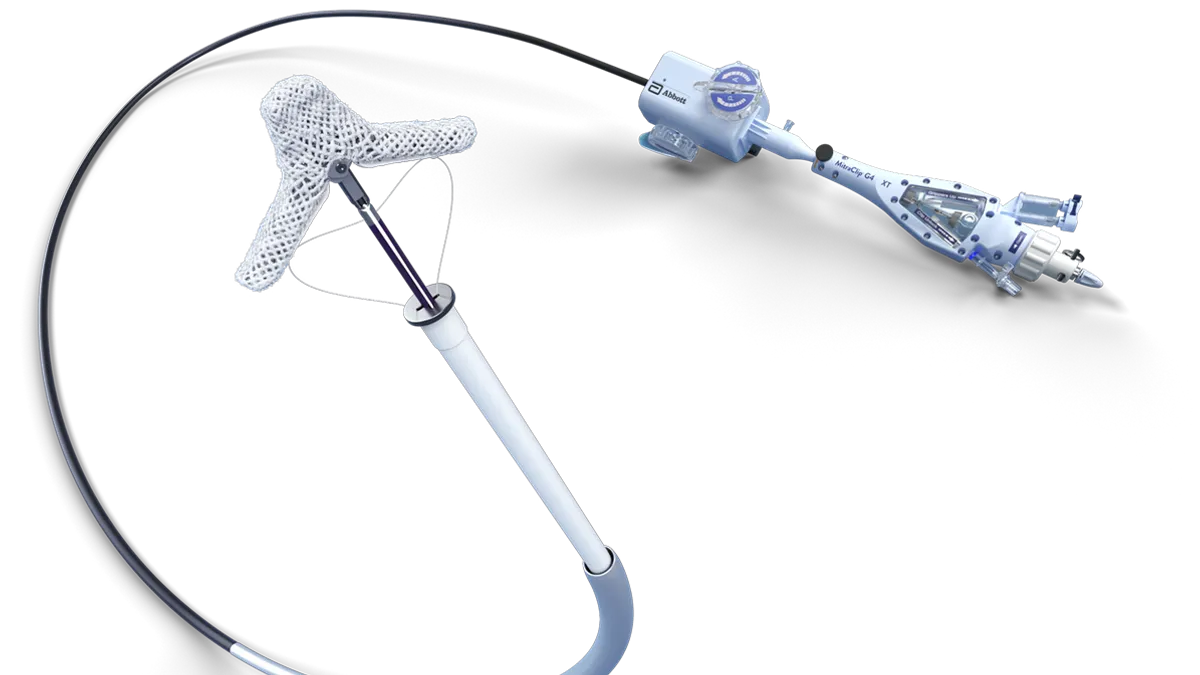
FDA alerts physicians to lock malfunction with Abbott’s MitraClip heart valve device
The problem, which the FDA said occurs in 1.3% of procedures, can have consequences including the need for surgery to treat significant residual disease.
By Nick Paul Taylor • Sept. 9, 2022 -
FDA: Reports of squamous cell carcinoma, lymphomas in scar tissue around breast implants
The report adds to the history of product safety problems for breast implant devices, including patient injuries and deaths.
By Ricky Zipp • Sept. 8, 2022 -

Website of medical device sales form: https://spwindustrial.com/baxter-sigma-spectrum-infusion-pump-s-w-ver-8-with-dual-antenna-wireless-batt/?gclid=Cj0KCQjwpeaYBhDXARIsAEzItbFttwA55ImqoesvcmNYfm6od_td4SGgOeNOqFOQohCiU_ci1yN-oHsaAqiFEALw_wcB

Cybersecurity firm finds vulnerabilities in Baxter’s Sigma infusion pumps
The weaknesses could allow attackers to access Wi-Fi data and make the device unavailable.
By Nick Paul Taylor • Updated Sept. 8, 2022 -
French prosecutors investigating Philips Respironics recall: Reuters
In addition to a U.S. DOJ inquiry, Philips now faces an investigation from French prosecutors over its recall of sleep apnea machines and ventilators.
By Elise Reuter • Sept. 8, 2022 -
Abbott heart device for newborns shows longer-term benefits with three-year data
The trial results give Abbott more evidence as it seeks to position the product as an alternative to medical management or surgical ligation.
By Nick Paul Taylor • Sept. 8, 2022 -
FDA clears Magnus neuromodulation system in major depressive disorder
Almost 80% of people treated with the SAINT system entered remission, compared to 13% of their peers in the control group.
By Nick Paul Taylor • Sept. 7, 2022 -
Philips recalls 17M sleep apnea masks over magnets that could affect implanted devices
The problem has caused 14 serious injuries, and the FDA cautioned that the issue could lead to deaths.
By Nick Paul Taylor • Updated Sept. 8, 2022 -
European Commission blocks Illumina-Grail deal to ‘preserve competition’ in cancer test market
The EC decision comes after a U.S. ruling to let the acquisition move forward and leaves Illumina facing an uncertain future in a competitive market for next-generation gene tests to detect cancer.
By Ricky Zipp • Sept. 6, 2022 -
Medtronic recalls endotracheal tubes after reports of airway obstruction
The recall includes certain brands of Medtronic’s EMG endotracheal tubes and affects more than 600,000 devices worldwide.
By Elise Reuter • Sept. 2, 2022 -
Q&A
Friday Q&A: iRhythm CTO Mark Day talks Zio Watch, Verily partnership, Big Tech in healthcare
Day spoke about developing a smartwatch for cardiac monitoring with Alphabet’s Verily, competing with Apple and building clinical evidence for the new product.
By Ricky Zipp • Sept. 2, 2022 -
Vibrating, colon-stimulating capsule to treat chronic constipation approved by FDA
Vibrant Gastro’s drug-free capsule increased complete spontaneous bowel movements in a pivotal clinical trial, opening a new treatment pathway for the millions of Americans suffering from severe constipation.
By Nick Paul Taylor • Sept. 2, 2022 -
Philips pays $24M to settle second set of claims by US Justice Dept.
Respironics allegedly purchased, “at a significant cost to itself,” data on the prescribing decisions of U.S. physicians and gave the information to durable medical equipment suppliers so that they would promote its products.
By Nick Paul Taylor • Sept. 2, 2022 -
Judge rules in favor of Illumina-Grail merger, countering FTC concerns
A ruling by a federal administrative judge makes it more likely Illumina can keep control of its one time subsidiary, which makes cancer tests. A European Union anti-competition review is still pending.
By Ricky Zipp , Elise Reuter • Updated Sept. 2, 2022 -
Medtronic grows patient monitoring offerings with BioIntelliSense partnership
Wearable patient monitors can help with healthcare staffing shortages, an ongoing challenge for hospitals, and create an opportunity for device makers.
By Ricky Zipp • Sept. 1, 2022 -
Baxter gets 510(k) clearance for syringe pump, spurring optimism of faster FDA review process
Syringe pumps are a potential new driver of growth for Baxter, and analysts at J.P. Morgan called the clearance “a nice win for the company,” as FDA timelines for pumps more broadly have remained “a challenge” for medtech companies.
By Nick Paul Taylor • Sept. 1, 2022 -
 Retrieved from Integra Life on August 24, 2022
Retrieved from Integra Life on August 24, 2022
Malfunctioning Integra intracranial monitor was used on patient who died: FDA
The team caring for the patient had to replace the sensor in a malfunctioning monitor, but Integra determined that the death was unrelated to the device, the agency said.
By Nick Paul Taylor • Sept. 1, 2022