FDA: Page 61
-

 The image by Gage Skidmore is licensed under CC BY-SA 2.0
The image by Gage Skidmore is licensed under CC BY-SA 2.0
Biden transition team names COVID-19 advisers, seeks dramatic testing scale-up with federal approach
The president-elect calls for doubling drive-through test sites and increasing capacity by "orders of magnitude" through investing in rapid at-home tests. The transition team has already called in help from former FDA and BARDA heads.
By Greg Slabodkin • Nov. 9, 2020 -

 "200323-Z-IB607-0016" by New Jersey National Guard is licensed under CC BY-ND 2.0
"200323-Z-IB607-0016" by New Jersey National Guard is licensed under CC BY-ND 2.0
GenScript wins 1st FDA nod to test for antibodies that could neutralize COVID-19
The Hong Kong-listed biotech's offering differs from earlier EUAs for serology tests from Abbott, Roche and Siemens Healthineers that only screen for antibodies that do not necessarily cut viral infection.
By Susan Kelly • Nov. 9, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
FDA greenlights digital therapeutic for Apple Watch to quell PTSD nightmares
The device, from Minneapolis-based startup Nightware, monitors heart rate and body movement data to best deliver vibrations that interrupt a nightmare without waking the person sleeping.
By Susan Kelly • Nov. 9, 2020 -
Even if Biden wins, divided Congress stifles chance for more progressive health policies
Results of the election are not final and may be uncertain for weeks, but the most likely scenario points to mostly incremental change, a positive for many parts of the healthcare sector.
By Shannon Muchmore • Nov. 5, 2020 -
FDA warns of COVID-19 antigen test false positives as report flags Quidel on accuracy
The agency alert, prompted by reports from nursing homes and other settings, comes a day after data emerged raising concerns about the ability of a Quidel test to detect asymptomatic cases.
By Nick Paul Taylor • Nov. 4, 2020 -
MedPAC pans CMS idea to lean on commercial payers for Medicare coverage decisions
The advisory group warned against threats to transparency and rigor in response to a proposed rule defining the term "reasonable and necessary" and adding a faster coverage pathway for FDA-designated breakthrough devices.
By Susan Kelly • Nov. 3, 2020 -
CMS finalizes rejection of Baxter, Outset dialysis device add-on payments
The broader, now final, ESRD rule is meant to support use of equipment and supplies for at-home dialysis treatment.
By Nick Paul Taylor • Updated Nov. 3, 2020 -
Duke researchers pitch CDS tool to keep electives going during COVID-19 surges
JAMA Network Open authors found that using predictive modeling to develop a clinical decision support tool could help determine patient length of stay and use of a ventilator in procedures like knee and hip replacements.
By Ron Shinkman • Nov. 2, 2020 -
CMS competitive bidding process fails to drive expected savings, sparking rethink
While Needham analysts contend one action removes an overhang for Inogen, Invacare and ResMed, those at Jefferies see a "major negative" in the agency's determination to lower rates outlined in a proposed rule.
By Nick Paul Taylor • Oct. 29, 2020 -
Neovasc angina device fails to win FDA panel backing, stock tumbles 43%
By a vote of 17-1, experts on FDA's circulatory system devices panel said the available data did not provide reasonable assurance of effectiveness, though most thought the Reducer system was safe for patients.
By Susan Kelly • Oct. 28, 2020 -


Yujin Kim / MedTech Dive, original photo courtesy of U.S. Food and Drug Administration

MDUFA V talks kick off as FDA grapples with onslaught of COVID-19 submissions
Medtech industry groups broadly expressed a desire to maintain the status quo after FDA Commissioner Stephen Hahn described the strain on agency workers under MDUFA IV as unsustainable.
By Maria Rachal • Oct. 28, 2020 -
USPSTF proposes lowering colorectal cancer screening age in boost to Exact Sciences' Cologuard
A federal task force, which has recommended the stool-based kit, now advises screening starting at age 45 after modeling found the change would avert one additional death per 1,000 adults.
By Nick Paul Taylor • Oct. 28, 2020 -
Neovasc refractory angina treatment faces FDA panel review
The agency's ultimate approval decision on the Canadian company's CE-marked device has important financial implications for the medtech, which saw its revenue plunge and operating loss deepen in the second quarter.
By Susan Kelly • Oct. 27, 2020 -
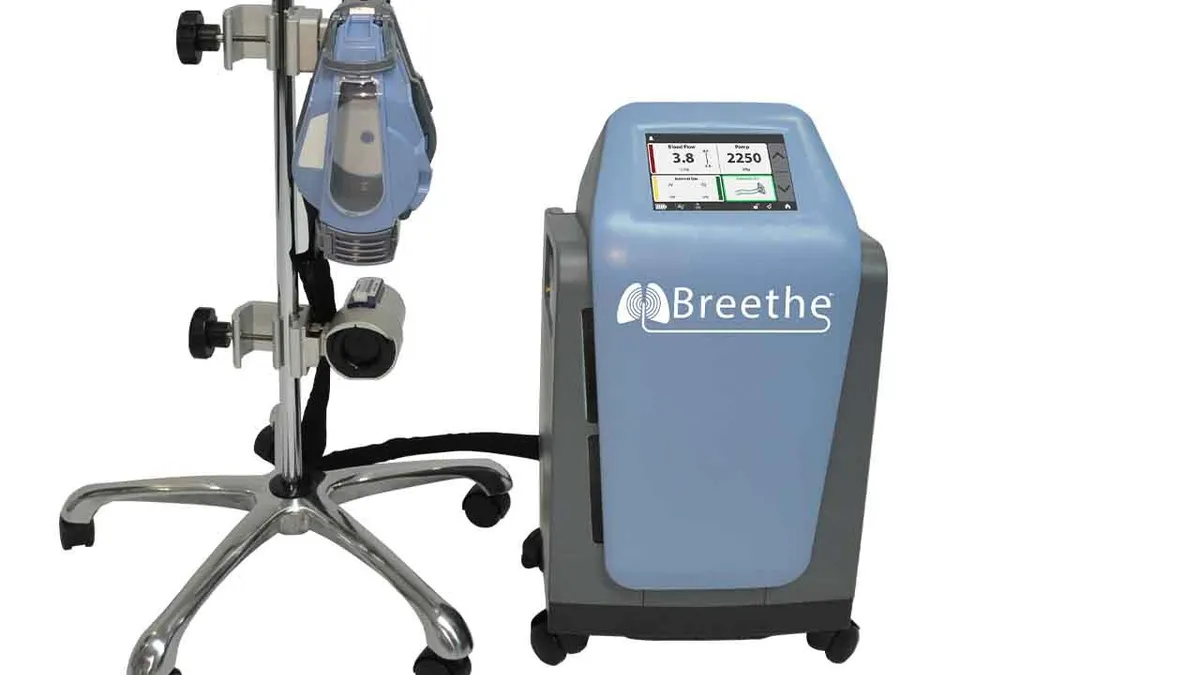
 Retrieved from Abiomed/BusinessWire on April 30, 2020
Retrieved from Abiomed/BusinessWire on April 30, 2020
FDA grants 510(k) to Abiomed's artificial lung, teeing up use in COVID-19
While LivaNova and Medtronic already sell ECMO devices, Abiomed is focusing on a relatively compact design to differentiate the device from the pack.
By Nick Paul Taylor • Oct. 27, 2020 -
FDA, Philips warn of data bias in AI, machine learning devices
The comments come 18 months after FDA unveiled a yet-to-be-finalized framework for modifying AI/ML-based software as a medical device using real-world learning and adaptation.
By Greg Slabodkin • Oct. 26, 2020 -
EU notified body designation pipeline points to IVDR bottleneck
An update from the European Commission reveals only a few notified bodies are likely to join the four already designated over the coming months.
By Nick Paul Taylor • Oct. 26, 2020 -
HHS walks back CARES fund reporting requirement hospitals feared
Health systems worried they may lose COVID-19 relief money after guidance changed last month. Those funds have been crucial in mitigating big hits to capital spending, execs at medtechs like BD and Stryker have said.
By Samantha Liss • Oct. 23, 2020 -
Neuromod devices at the fore in latest FDA breakthrough designations
Liquid biopsies also stand out as an area where U.S. regulators are encouraging development and prioritizing review.
By Nick Paul Taylor • Oct. 23, 2020 -
FDA floats framework to message cybersecurity threats to patients
The proposal, which has the agency and industry sharing responsibility for making the information easy to find, comes as the risks to medical devices continue to grow.
By Nick Paul Taylor • Oct. 21, 2020 -
AdvaMed seeks regulatory clarity on FDA's new digital health center
Director Bakul Patel says the new unit aims to provide the "least burdensome" oversight and that evidence will be key, but industry says medtechs still have questions on what rules they're subject to.
By Greg Slabodkin • Oct. 20, 2020 -
CMS coverage draft shuns 1st-to-market colorectal cancer blood test, outlines path for Exact, Guardant
The proposed Medicare coverage memo rejected Epigenomics' bid for payment, but Wall Street said the bar set was clearable by potential rival liquid biopsy developers working on similar products.
By Maria Rachal • Oct. 20, 2020 -
Future of COVID-19 products, device shortages, CDS top CDRH 2021 to-do list
Regulators aim to publish final guidance on five topics and draft documents on another dozen priority areas in the coming year. A few of the goals are carryovers as priorities shifted during the public health emergency.
By Susan Kelly • Oct. 19, 2020 -
CMS expands Medicare emergency telehealth coverage with 11 new services
For the first time in five months, the agency made new virtual care additions including electronic analysis of an implanted neurostimulator pulse generator and intensive cardiac rehab with or without continuous EKG monitoring.
By Rebecca Pifer Parduhn • Oct. 16, 2020 -
CMS to cut COVID-19 test pay by 25% for delayed results, labs cry foul
The American Clinical Laboratory Association, which includes LabCorp and Quest, argued the new policy fails to address the root of delays: fluctuating demand and supply chain disruptions.
By Nick Paul Taylor • Oct. 16, 2020 -
FDA finalizes guidance on nitinol devices, posts biocompatibility draft
The agency has been looking at the alloy, commonly used in stents and heart valves, as part of a broader look at metals that can cause inflammatory or other types of reactions once inside the body.
By Nick Paul Taylor • Oct. 15, 2020