Medical Devices: Page 128
-
Ortho device reclassifications, MDUFA reauthorization among FDA's fall meeting lineup
The agency's upcoming slate of virtual medtech meetings kicks off Tuesday with an orthopaedic devices panel that will consider a proposal to downclassify noninvasive bone growth stimulators.
By Susan Kelly • Sept. 8, 2020 -
Surgeries keep rising, buoying Wall Street's year-end medtech outlook
Jefferies reported a fourth consecutive week of growing hospital traffic, while Bank of America is "broadly bullish" on the medical devices sector as 2020 wraps up.
By Greg Slabodkin • Sept. 8, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
Edwards gets FDA approval for Sapien 3 in new patient population
The PMA expands the transcatheter valve for use in congenital heart defect patients who suffer problems following surgical repair, positioning the device to compete against a range of treatment options.
By Nick Paul Taylor • Sept. 8, 2020 -
Abbott resurrects resorbable scaffold tech for below-the-knee trial
The medtech previously stopped selling the technology amid evidence it increased the risk of major adverse cardiac events, but recent data has revealed a new BTK opportunity.
By Nick Paul Taylor • Sept. 4, 2020 -
FDA flags 428 spinal cord stimulator patient deaths, urges more tests before implant
The agency revealed the figures in a letter to healthcare providers urging simulations prior to permanently implanting the devices, sold by companies such as Abbott, Boston Scientific, Medtronic and Nevro.
By Nick Paul Taylor • Sept. 4, 2020 -
Watchdog gives high marks to CMS competitive bidding program
The Office of Inspector General probe found the agency followed procedures 97% of the time when awarding contracts for durable medical equipment, prosthetics, orthotics and supplies.
By Nick Paul Taylor • Sept. 3, 2020 -
Boston Scientific, Stryker win CMS new tech add-on pay, BD left out
When combining eligible devices with other recipients, including drug therapies, the agency estimates spending on the payments will total roughly $874 million, a 120% year-over-year increase.
By Maria Rachal • Sept. 2, 2020 -
Stryker reaches agreement with Colfax for Wright Medical deal divestitures
The medtech disclosed Thursday the orthopaedics competitor's DJO Global subsidiary agreed to acquire its ankle and finger joint replacement businesses to shore up U.S. and U.K. antitrust concerns.
By Maria Rachal • Updated Oct. 16, 2020 -
HHS terminates Hamilton, Vyaire ventilator contracts
The Strategic National Stockpile now has an adequate supply of the devices, the agency said. The two companies will not deliver the additional 38,000 ventilators they'd planned by year's end.
By Greg Slabodkin • Sept. 2, 2020 -
UK outlines post-Brexit medical device regime starting January
Regulators unveiled a future U.K. Conformity Assessed (UKCA) mark, but will continue recognizing CE marks until mid-2023 and give companies four to 12 months to register their devices and in vitro diagnostics.
By Nick Paul Taylor • Sept. 2, 2020 -
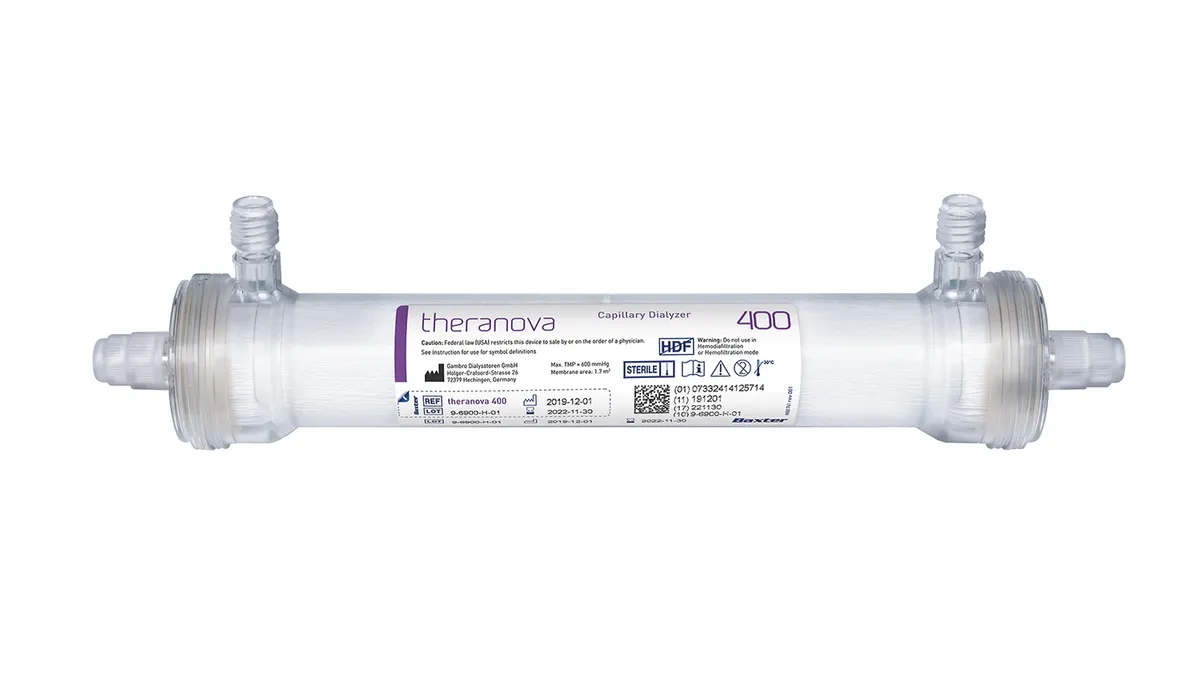
Baxter wins FDA De Novo for more expansive dialyzer
The medtech is still working on convincing CMS to support add-on Medicare payments for the devices.
By Maria Rachal • Sept. 1, 2020 -
Medtronic artificial pancreas gets FDA nod for young kids
The medtech is introducing MiniMed 770G, a Bluetooth-enabled version of the first hybrid closed looped system to get agency approval, bridging the gap to a device launching in Europe this fall but delayed in the U.S.
By Nick Paul Taylor • Sept. 1, 2020 -
CMS pitches coverage of breakthrough devices in tandem with FDA authorization
The proposal follows years of AdvaMed lobbying for products awarded the special FDA designation to gain Medicare reimbursement upon clearance or approval.
By Maria Rachal , Susan Kelly • Aug. 31, 2020 -
FDA proposes adding patient voice in device evaluation
The draft guidance comes nearly two years after an advisory committee prioritized greater inclusion of the patient perspective in device design, development and assessment. Two more public meetings are planned.
By Susan Kelly • Aug. 31, 2020 -
Philips lowers 2020 outlook as HHS nixes ventilator contract
The medtech received a "partial termination" notice and will not deliver 30,700 remaining ventilators to the Strategic National Stockpile, a month after a House panel called for a probe into the roughly $647 million contract.
By Greg Slabodkin • Aug. 31, 2020 -
EU MDR costing smaller medtechs 5% of their annual sales: survey
Almost 70% of 101 companies surveyed by a German software provider said they spend most of their time related to the new rules deciphering what the incoming EU Medical Device Regulation means.
By Nick Paul Taylor • Aug. 31, 2020 -
Stryker stretches Wright Medical share offer into Q4
As the medtech makes the fifth offer extension since the $4 billion acquisition was announced last November, the company is approaching the latest time it said the deal would close.
By Susan Kelly • Updated Sept. 29, 2020 -
Clinical trial links hospital use of Dexcom G6 to improved glucose control
Scripps Whittier Diabetes Institute researchers found the sensors safe and effective in the hospital setting, newly cleared by FDA amid the pandemic. Whether the benefits justify the additional cost is unclear.
By Nick Paul Taylor • Aug. 28, 2020 -
Philips pens $275M Intact Vascular deal to acquire implant
The buy adds the first FDA-approved vascular implant for below-the-knee interventions to the Dutch conglomerate's portfolio.
By Nick Paul Taylor • Aug. 28, 2020 -
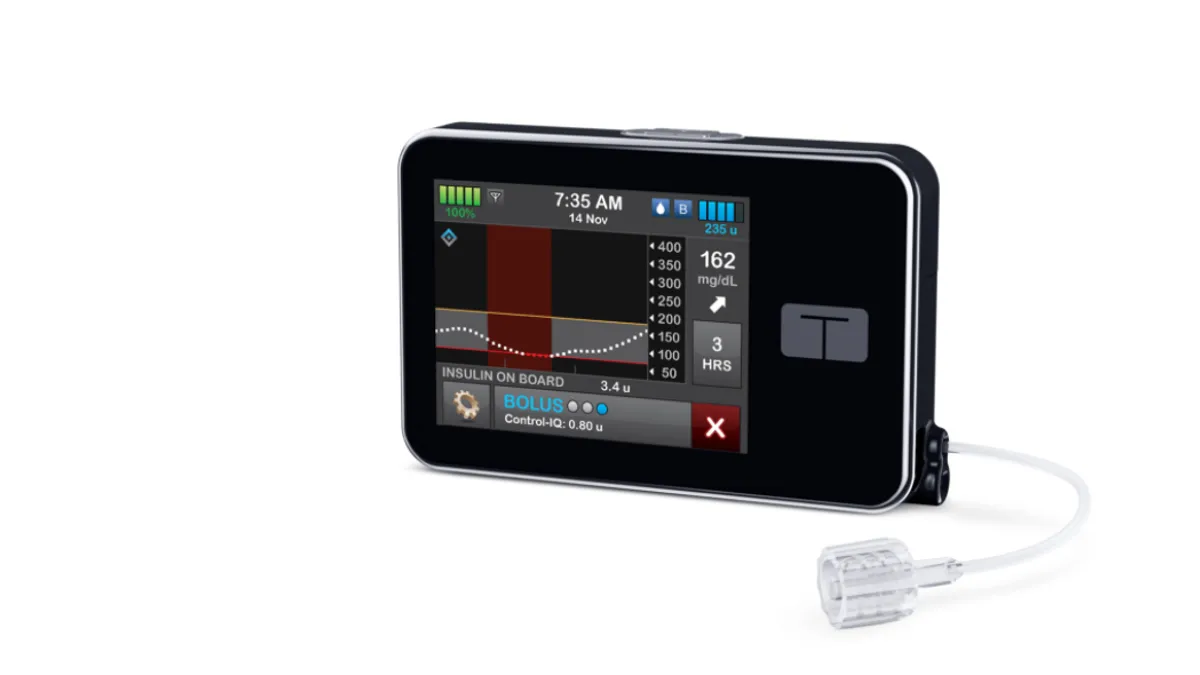
Tandem's artificial pancreas improves glucose control in pediatric trial
Children treated with the closed-loop insulin delivery system spent longer in the target glucose range than peers who just received Dexcom G6 sensors and insulin, the study published in the New England Journal of Medicine found.
By Nick Paul Taylor • Aug. 27, 2020 -
With a one-year reprieve to EU's MDR, some procrastinate, others speed ahead
Industry is taking advantage of a long-sought delay to the regulation in different ways, but COVID-19's impact on notified body capacity, pace of information from lawmakers, and company resources are some confounding variables.
By Maria Rachal • Aug. 26, 2020 -
Trump admin delays final rule easing anti-kickback regs until next August
Devicemakers back proposed changes to the law, passed decades ago to deter physicians from referring patients to locations that give them a financial benefit, calling them outdated and burdensome.
By Rebecca Pifer Parduhn • Aug. 26, 2020 -
Hologic inks $80M Acessa buyout to expand women's health business
The cash acquisition, plus contingent payments based on future revenue growth, will add a treatment for benign uterine fibroid to the medtech's portfolio.
By Nick Paul Taylor • Aug. 26, 2020 -
Insulin pumps among millions of devices facing risk from newly disclosed cyber vulnerability, IBM says
The firm's hacking team said the vulnerability may allow criminals to remotely alter patient dosing, as well as manipulate readings from medical device monitors "to cover up concerning vital signs or create false panic."
By Greg Slabodkin • Aug. 25, 2020 -
Medtronic's Martha teases focus shift on diabetes, robotics
After pandemic-driven delays to its soft tissue robot, the company also told investors it expects to file for a CE mark and a U.S. investigational device exemption in the first quarter of calendar year 2021.
By Maria Rachal • Aug. 25, 2020