Medical Devices: Page 129
-
FDA-industry group touts real-world evidence framework to speed test development
The Medical Device Innovation Consortium, a collaboration between FDA and diagnostics makers like Abbott and Roche, offers a roadmap as COVID-19 accelerates use of newly generated data to update emergency authorizations.
By Susan Kelly • Aug. 25, 2020 -
Q&A
MedTech Europe beats drum for virtual audits, IVDR delay
The one-year delay to the EU Medical Device Regulation has been crucial, but there's been "radio silence" from lawmakers on calls for a similar delay to the In Vitro Diagnostic Regulation, said regulatory lead Oliver Bisazza.
By Maria Rachal • Aug. 24, 2020 -
 Explore the Trendline➔
Explore the Trendline➔
 Permission granted by Boston Scientific
Permission granted by Boston Scientific Trendline
TrendlineNew medical devices are reshaping the medtech industry
From pulsed field ablation devices to glucose sensors and surgical robotics, new medical technologies are transforming patient care and how people manage their health.
By MedTech Dive staff -
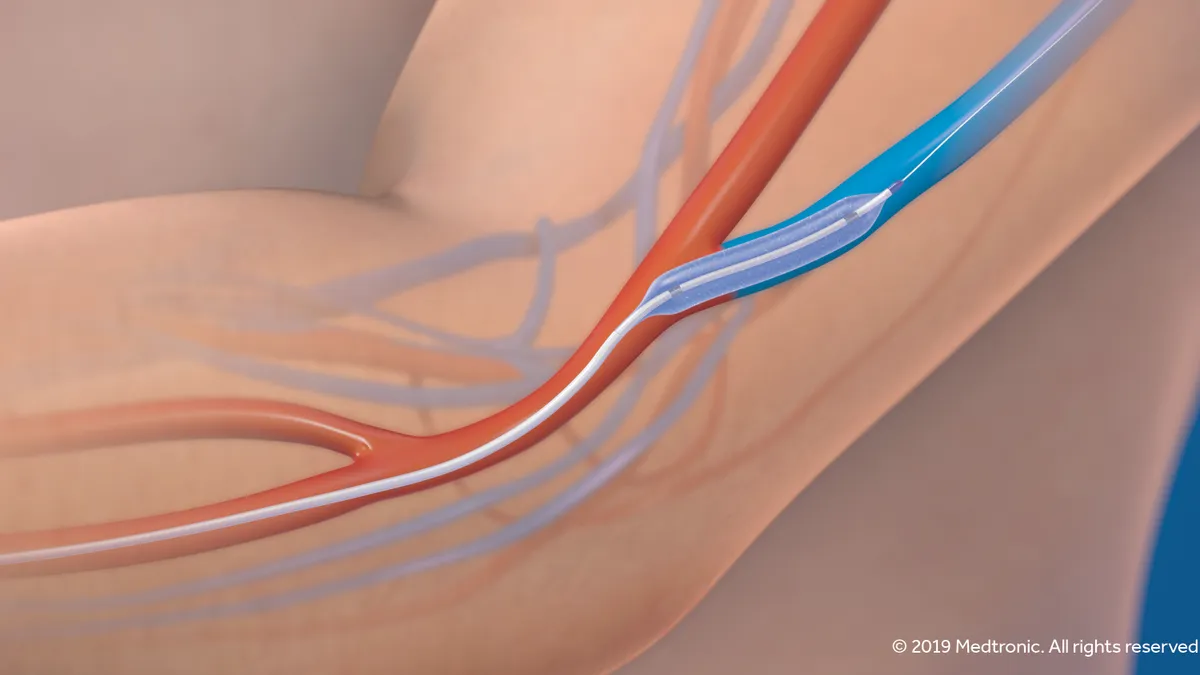
Medtronic hopes for renewed spotlight on dialysis access device with NEJM publication
The company-funded study in the New England Journal of Medicine comes amid widespread disruption of vascular access procedures, and nine months after Medtronic got FDA approval for its IN.PACT AV drug-coated balloon.
By Maria Rachal • Aug. 21, 2020 -
Intuitive robot may up survival in mouth and throat cancers, but not others: study
Researchers in JAMA Oncology said the findings back a randomized clinical trial for oropharyngeal patients. The da Vinci system had no effect on overall survival in patients with prostate, endometrium and cervix cancers.
By Nick Paul Taylor • Aug. 21, 2020 -
Eudamed's first section to go live before year's end
The European Commission is ready to move on the initial module of the new safety and performance database, created as part of the incoming Medical Device Regulation, ahead of a March 2021 deadline.
By Nick Paul Taylor • Aug. 19, 2020 -
CMS urges resumption of essential procedures, organ transplants for ESRD patients
Medicare beneficiaries with end-stage renal disease have 3.5 times heightened risk of COVID-19 infection, according to an HHS analysis of early claims data.
By Maria Rachal • Aug. 18, 2020 -
FEMA pitches voluntary DPA pact to bolster medical supply chain during pandemic
The Defense Production Act allows private companies to enter agreements with the government while avoiding antitrust liability. AdvaMed wants to participate but FEMA has not yet extended an invite.
By Greg Slabodkin • Aug. 18, 2020 -
Medtronic seeks leg up in lagging pen market with Companion Medical buy
As Dexcom and Abbott grow share in the continuous glucose monitoring market and Insulet and Tandem forge on with interoperable insulin pump systems, Medtronic is hoping to jump ahead with a connected pen.
By Maria Rachal • Aug. 17, 2020 -
FDA looks to downclass noninvasive bone growth stimulators, incumbents not on board
The agency made a case for moving the devices to less-rigorous Class II regulation in a proposal opposed by manufacturers with products already on the market such as Orthofix.
By Susan Kelly • Aug. 17, 2020 -
Surgical robots, early cancer tests top CB Insights digital health startup list
The most promising, as aggregated in annual rankings from the firm, have raised over $20 billion in total funding for more than 600 deals with 900-plus investors.
By Hailey Mensik , Maria Rachal • Aug. 14, 2020 -
Hospitals, docs pitch roadmap to keep essential surgeries going even with coronavirus surges
While many medtechs cast the worst of the pandemic's impact in the rearview mirror in recent financial reports, concerns from healthcare industry groups reflect a more nuanced picture.
By Nick Paul Taylor • Aug. 14, 2020 -
CMS seeks to ease rules on artificial heart, ventricular assist device coverage
Potential eligibility changes for advanced heart failure patients drew positive feedback from Abbott and Medtronic, while removal of a coverage with evidence development policy has been opposed by several cardiologist groups.
By Maria Rachal • Aug. 13, 2020 -
Watchdog again pushes CMS to add UDIs to claim forms
The HHS Office of Inspector General said that without a place to ask for device-specific information, it's difficult for the agency to track billions of dollars in costs related to failed devices.
By Nick Paul Taylor • Aug. 13, 2020 -
Coronavirus muddies FDA's trajectory on device warning letters
An agency official said going into 2020 that its devices center would be better equipped to use the compliance tool in a timely fashion. But a dry spell for on-site surveillance may undermine any noticeable rebound.
By Maria Rachal • Updated Aug. 12, 2020 -
Inari Medical revenue rises in 1st quarterly report since public debut
Specializing in clot-removal devices for pulmonary embolism and deep vein thrombosis, the medtech's CEO downplayed new competition in the space, claiming that only 2% of the $3.6 billion market is penetrated.
By Susan Kelly • Aug. 12, 2020 -
FDA posts Essure adverse events pulled from social media
The spreadsheet includes details of 53 deaths and 1,376 reports of serious injuries related to the controversial birth control implant but the agency is unsure if it contains duplicate reports.
By Nick Paul Taylor • Updated Aug. 14, 2020 -
Medtronic to buy Companion Medical, adding connected insulin pen to diabetes unit
Building on tuck-in acquisitions to a business losing market share in recent years, the deal gives the medtech giant an FDA-cleared device for people with Type 1 or Type 2 diabetes on multiple daily injection therapy.
By Greg Slabodkin • Aug. 11, 2020 -
Sientra claims market gains as breast implant sales rebound faster than rivals
AbbVie's Allergan and Establishment Labs saw deeper declines in second quarter financials. Sientra CEO Jeffrey Nugent boasted the company is "clearly taking share" from rivals and is hiring to meet demand.
By Nick Paul Taylor • Aug. 11, 2020 -
Struggling Senseonics allies with blood glucose monitoring giant Ascensia, taps much-needed $80M
Senseonics has failed to dent a market largely commanded by Abbott and Dexcom, despite having a product differentiated from its non-implantable competitors. Analysts believe the new commercialization pact will help.
By Nick Paul Taylor • Aug. 11, 2020 -
BD's FDA filing to update recalled Alaris pumps delayed by 6 months
The medtech planned to rectify the situation with FDA this year, but that schedule has slipped as it continues system testing and ongoing discussions with the agency. BD estimates remediation costs will total $200 million.
By Greg Slabodkin • Aug. 10, 2020 -
Notified bodies list for incoming EU regs hits 20 with designation of DQS for MDR
The 16 notified bodies now allowed to complete work under the Medical Device Regulation and the four OK'd under its In Vitro Diagnostic counterpart together meet the goal that authorities had hoped to achieve by the start of 2020.
By Nick Paul Taylor • Aug. 10, 2020 -
Healthcare gains jobs in July, but still down 800K from February
The latest report shows ambulatory, outpatient, and medical and diagnostic lab service jobs are creeping closer to last year's levels.
By Hailey Mensik • Aug. 7, 2020 -
Insulet tops Q2 forecasts, as diabetes tech resilient in wake of coronavirus
The number of new insulin pump users was halved in the quarter as COVID-19 reduced typical patient-doctor interactions, but the company kept its $1 billion revenue target for next year.
By Maria Rachal • Aug. 7, 2020 -
Acutus raises $159M in IPO, challenging medtech leaders for cardiac mapping market
The arrhythmia management company's transition is part of its strategy to crack a market already being fought over by medtech giants Abbott, Boston Scientific, Johnson & Johnson and Medtronic.
By Nick Paul Taylor • Aug. 7, 2020 -
BD sales slide below expectations, as COVID-19 antigen test gains momentum
In the month since the launch of its antigen test, CEO Tom Polen said BD shipped more Veritor readers than in a typical year. Still, the medtech's stock fell 9% Thursday amid pressure on other business lines.
By Greg Slabodkin • Aug. 6, 2020